The glucosinolate-myrosinase system. New insights into enzyme-substrate interactions by use of simplified inhibitors.
Bourderioux, A., Lefoix, M., Gueyrard, D., Tatibouet, A., Cottaz, S., Arzt, S., Burmeister, W.P., Rollin, P.(2005) Org Biomol Chem 3: 1872-1879
- PubMed: 15889170
- DOI: https://doi.org/10.1039/b502990b
- Primary Citation of Related Structures:
1W9B, 1W9D - PubMed Abstract:
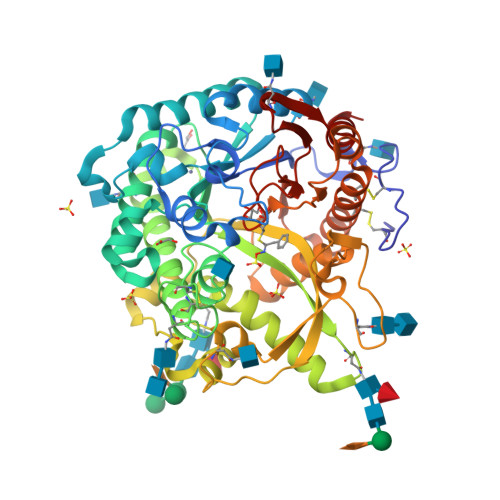
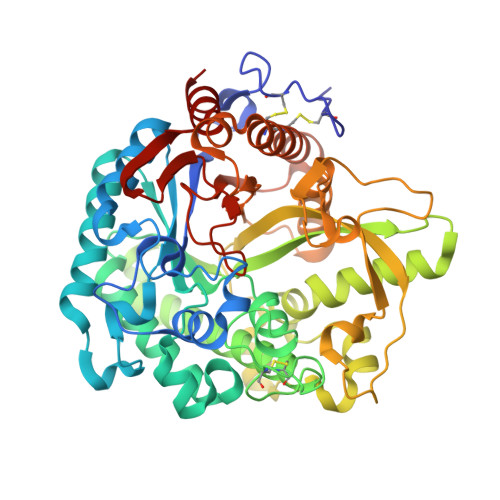
Myrosinase, a thioglucoside glucohydrolase, is the only enzyme able to hydrolyse glucosinolates, a unique family of molecules bearing an anomeric O-sulfated thiohydroximate function. Non-hydrolysable myrosinase inhibitors have been devised and studied for their biological interaction. Diverse modifications of the O-sulfate moiety did not result in a significant inhibitory effect, whereas replacing the D-glucopyrano residue by its carba-analogue allowed inhibition to take place. X-Ray experiments carried out after soaking allowed for the first time inclusion of a non-hydrolysable inhibitor inside the enzymatic pocket. Structural tuning of the aglycon part in its pocket is being used as a guide for the development of simplified and more potent inhibitors.
Organizational Affiliation:
Institut de Chimie Organique et Analytique (ICOA), UMR 6005, Université d'Orléans, BP 6759, F-45067, Orléans Cedex 2, France.