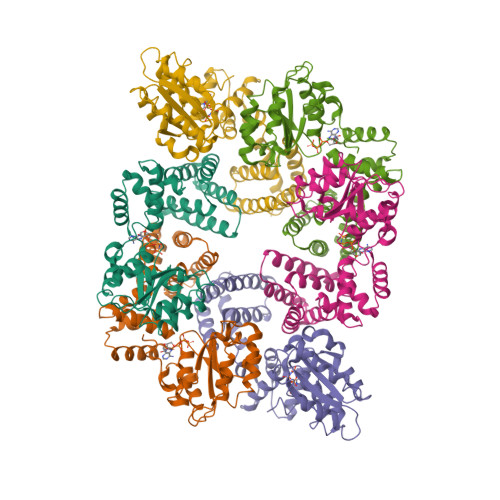
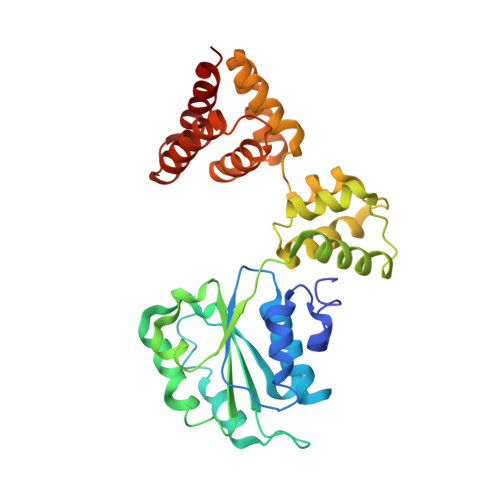
Communication between Subunits within an Archaeal Clamp-Loader Complex.
Seybert, A., Singleton, M.R., Cook, N., Hall, D.R., Wigley, D.B.(2006) EMBO J 25: 2209
- PubMed: 16628222
- DOI: https://doi.org/10.1038/sj.emboj.7601093
- Primary Citation of Related Structures:
2CHG, 2CHQ, 2CHV - PubMed Abstract:
We have investigated the communication between subunits in replication factor C (RFC) from Archaeoglobus fulgidus. Mutation of the proposed arginine finger in the small subunits results in a complex that can still bind ATP but has impaired clamp-loading activity, a process that normally only requires binding of nucleotide. The small subunit alone forms a hexameric ring that is six-fold symmetric in the absence of ATP. However, this symmetry is broken when the nucleotide is bound to the complex. A conformational change associated with nucleotide binding may relate to the opening of PCNA rings by RFC during the loading reaction. The structures also reveal the importance of the N-terminal helix of each subunit at the ATP-binding site. Analysis of mutant protein complexes containing subunits lacking this N-terminal helix reveals key distinct regulatory roles during clamp loading that are different for the large and small subunits in the RFC complex.
Organizational Affiliation:
Clare Hall Laboratories, Cancer Research UK, London Research Institute, South Mimms Potters Bar, Herts, UK.