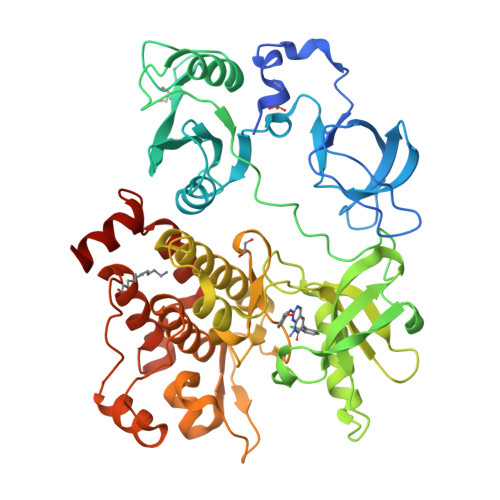
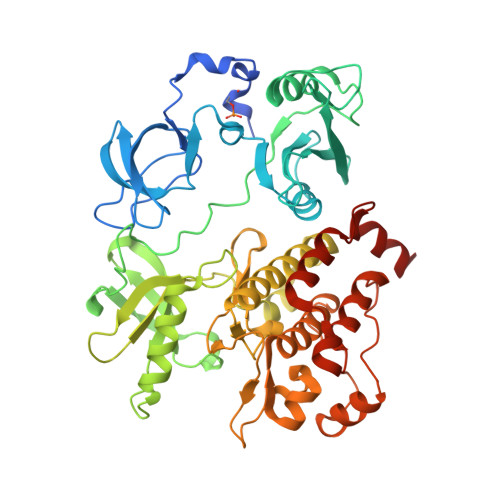
Organization of the SH3-SH2 unit in active and inactive forms of the c-Abl tyrosine kinase.
Nagar, B., Hantschel, O., Seeliger, M., Davies, J.M., Weis, W.I., Superti-Furga, G., Kuriyan, J.(2006) Mol Cell 21: 787-798
- PubMed: 16543148
- DOI: https://doi.org/10.1016/j.molcel.2006.01.035
- Primary Citation of Related Structures:
2FO0 - PubMed Abstract:
The tyrosine kinase c-Abl is inactivated by interactions made by its SH3 and SH2 domains with the distal surface of the kinase domain. We present a crystal structure of a fragment of c-Abl which reveals that a critical N-terminal cap segment, not visualized in previous structures, buttresses the SH3-SH2 substructure in the autoinhibited state and locks it onto the distal surface of the kinase domain. Surprisingly, the N-terminal cap is phosphorylated on a serine residue that interacts with the connector between the SH3 and SH2 domains. Small-angle X-ray scattering (SAXS) analysis shows that a mutated form of c-Abl, in which the N-terminal cap and two other key contacts in the autoinhibited state are deleted, exists in an extended array of the SH3, SH2, and kinase domains. This alternative conformation of Abl is likely to prolong the active state of the kinase by preventing it from returning to the autoinhibited state.
Organizational Affiliation:
Howard Hughes Medical Institute, Department of Molecular and Cell Biology, University of California, Berkeley, 94720, USA.