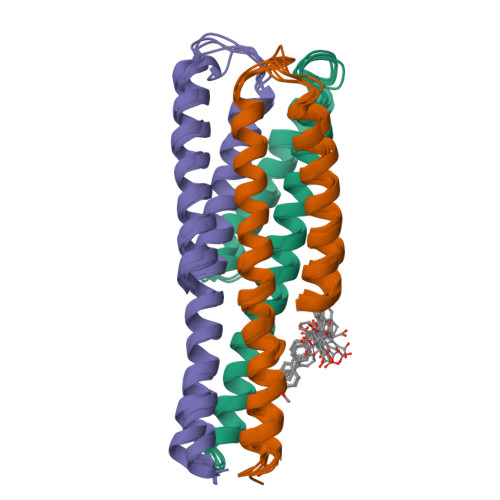
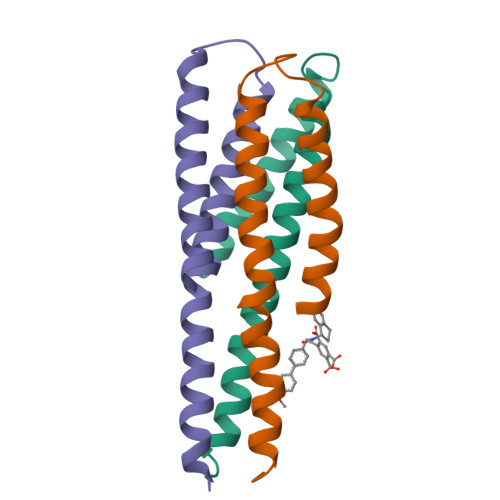
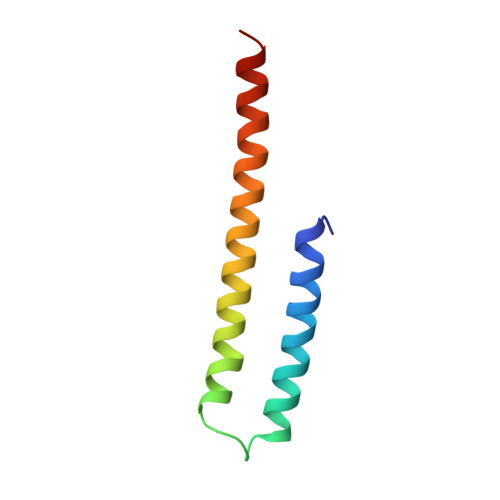
Non-peptide entry inhibitors of HIV-1 that target the gp41 coiled coil pocket.
Stewart, K.D., Huth, J.R., Ng, T.I., McDaniel, K., Hutchinson, R.N., Stoll, V.S., Mendoza, R.R., Matayoshi, E.D., Carrick, R., Mo, H., Severin, J., Walter, K., Richardson, P.L., Barrett, L.W., Meadows, R., Anderson, S., Kohlbrenner, W., Maring, C., Kempf, D.J., Molla, A., Olejniczak, E.T.(2010) Bioorg Med Chem Lett 20: 612-617
- PubMed: 20004576
- DOI: https://doi.org/10.1016/j.bmcl.2009.11.076
- Primary Citation of Related Structures:
2KP8 - PubMed Abstract:
The ectodomain of HIV-1 gp41 mediates the fusion of viral and host cellular membranes. The peptide-based drug Enfuvirtide(1) is precedent that antagonists of this fusion activity may act as anti HIV-agents. Here, NMR screening was used to discover non-peptide leads against this target and resulted in the discovery of a new benzamide 1 series. This series is non-peptide, low molecular weight, and analogs have activity in a cell fusion assay with EC50 values ranging 3-41microM. Structural work on the gp41/benzamide 1 complex was determined by NMR spectroscopy using a designed model peptide system that mimics an open pocket of the fusogenic form of the protein.
Organizational Affiliation:
Pharmaceutical Discovery Division, Abbott Laboratories, 100 Abbott Park Rd., R46Y, AP10, Abbott Park, IL 60064-6098, United States. kent.d.stewart@abbott.com