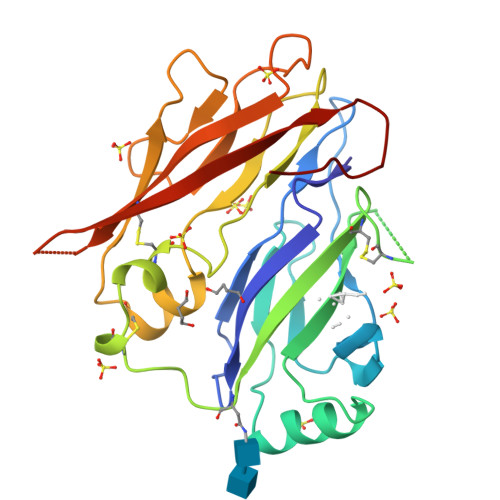
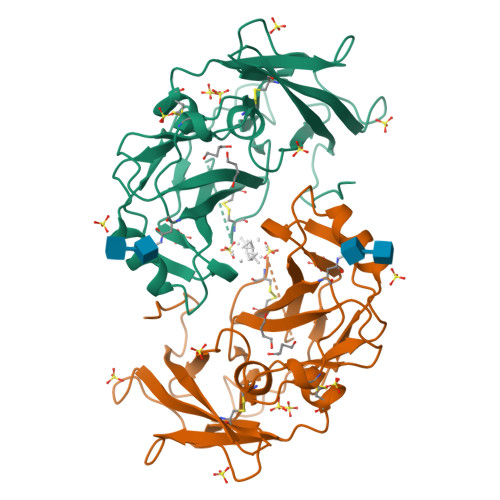
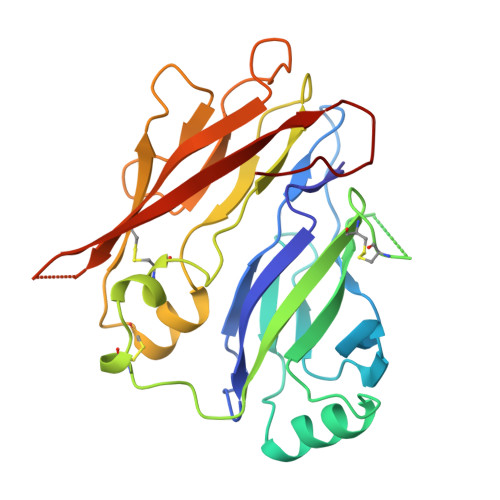
Mammal-restricted elements predispose human RET to folding impairment by HSCR mutations.
Kjaer, S., Hanrahan, S., Totty, N., McDonald, N.Q.(2010) Nat Struct Mol Biol 17: 726-731
- PubMed: 20473317
- DOI: https://doi.org/10.1038/nsmb.1808
- Primary Citation of Related Structures:
2X2U - PubMed Abstract:
The maturation of human RET is adversely affected by a range of missense mutations found in patients with Hirschsprung's disease (HSCR), a complex multigenic disease. Here we show that two N-terminal cadherin-like domains, CLD1 and CLD2 (CLD(1-2)), from human RET adopt a clam-shell arrangement distinct from that of classical cadherins. CLD1 structural elements and disulfide composition are unique to mammals, indicating an unexpected structural diversity within higher and lower vertebrate RET CLD regions. We identify two unpaired cysteines that predispose human RET to maturation impediments in the endoplasmic reticulum and establish a quantitative cell-based RET maturation assay that offers a biochemical correlate of HSCR disease severity. Our findings provide a key conceptual framework and means of testing and predicting genotype-phenotype correlations in HSCR.
Organizational Affiliation:
Structural Biology Laboratory, the London Research Institute, Cancer Research UK, London, UK.