Evolution of enzymatic activities in the enolase superfamily: L-rhamnonate dehydratase.
Rakus, J.F., Fedorov, A.A., Fedorov, E.V., Glasner, M.E., Hubbard, B.K., Delli, J.D., Babbitt, P.C., Almo, S.C., Gerlt, J.A.(2008) Biochemistry 47: 9944-9954
- PubMed: 18754693
- DOI: https://doi.org/10.1021/bi800914r
- Primary Citation of Related Structures:
2I5Q, 3BOX, 3CXO - PubMed Abstract:
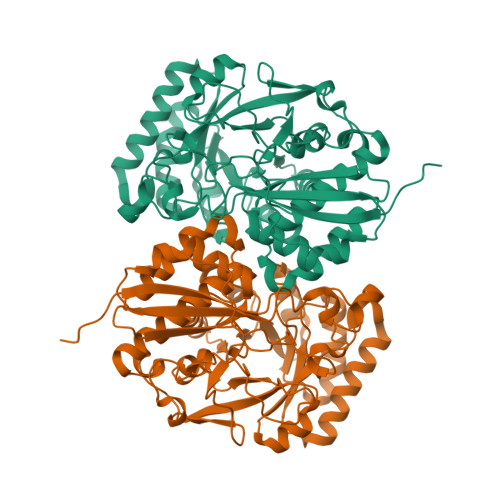
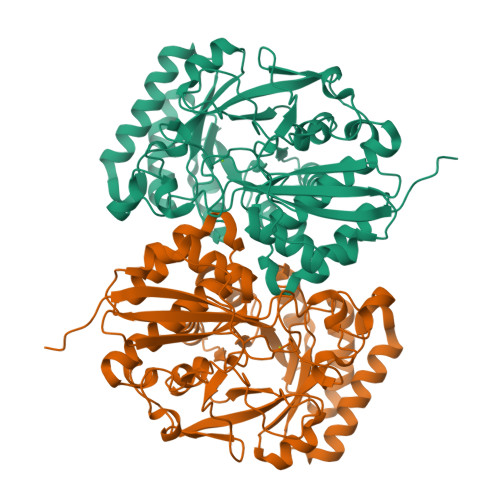
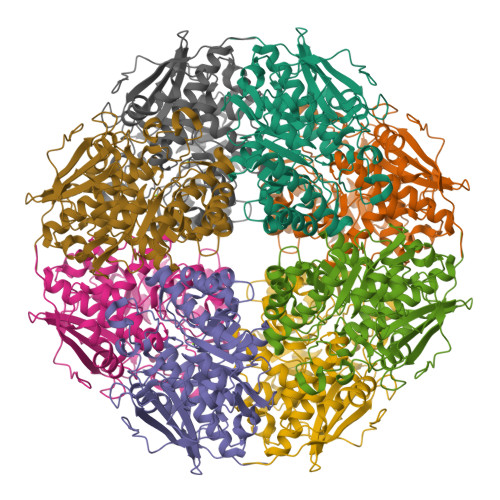
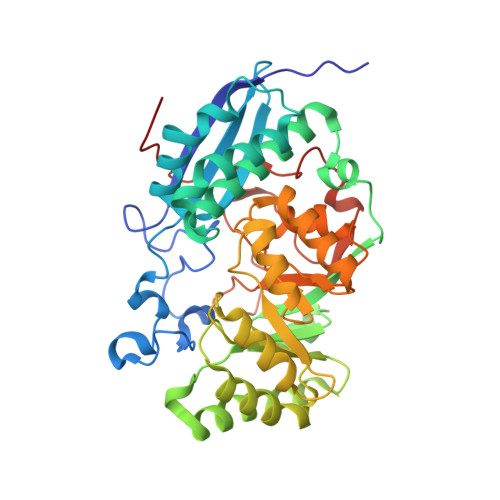
The l-rhamnonate dehydratase (RhamD) function was assigned to a previously uncharacterized family in the mechanistically diverse enolase superfamily that is encoded by the genome of Escherichia coli K-12. We screened a library of acid sugars to discover that the enzyme displays a promiscuous substrate specificity: l-rhamnonate (6-deoxy- l-mannonate) has the "best" kinetic constants, with l-mannonate, l-lyxonate, and d-gulonate dehydrated less efficiently. Crystal structures of the RhamDs from both E. coli K-12 and Salmonella typhimurium LT2 (95% sequence identity) were obtained in the presence of Mg (2+); the structure of the RhamD from S. typhimurium was also obtained in the presence of 3-deoxy- l-rhamnonate (obtained by reduction of the product with NaBH 4). Like other members of the enolase superfamily, RhamD contains an N-terminal alpha + beta capping domain and a C-terminal (beta/alpha) 7beta-barrel (modified TIM-barrel) catalytic domain with the active site located at the interface between the two domains. In contrast to other members, the specificity-determining "20s loop" in the capping domain is extended in length and the "50s loop" is truncated. The ligands for the Mg (2+) are Asp 226, Glu 252 and Glu 280 located at the ends of the third, fourth and fifth beta-strands, respectively. The active site of RhamD contains a His 329-Asp 302 dyad at the ends of the seventh and sixth beta-strands, respectively, with His 329 positioned to function as the general base responsible for abstraction of the C2 proton of l-rhamnonate to form a Mg (2+)-stabilized enediolate intermediate. However, the active site does not contain other acid/base catalysts that have been implicated in the reactions catalyzed by other members of the MR subgroup of the enolase superfamily. Based on the structure of the liganded complex, His 329 also is expected to function as the general acid that both facilitates departure of the 3-OH group in a syn-dehydration reaction and delivers a proton to carbon-3 to replace the 3-OH group with retention of configuration.
Organizational Affiliation:
Department of Biochemistry, University of Illinois at Urbana-Champaign, 600 South Mathews Avenue, Urbana, Illinois 61801, USA.