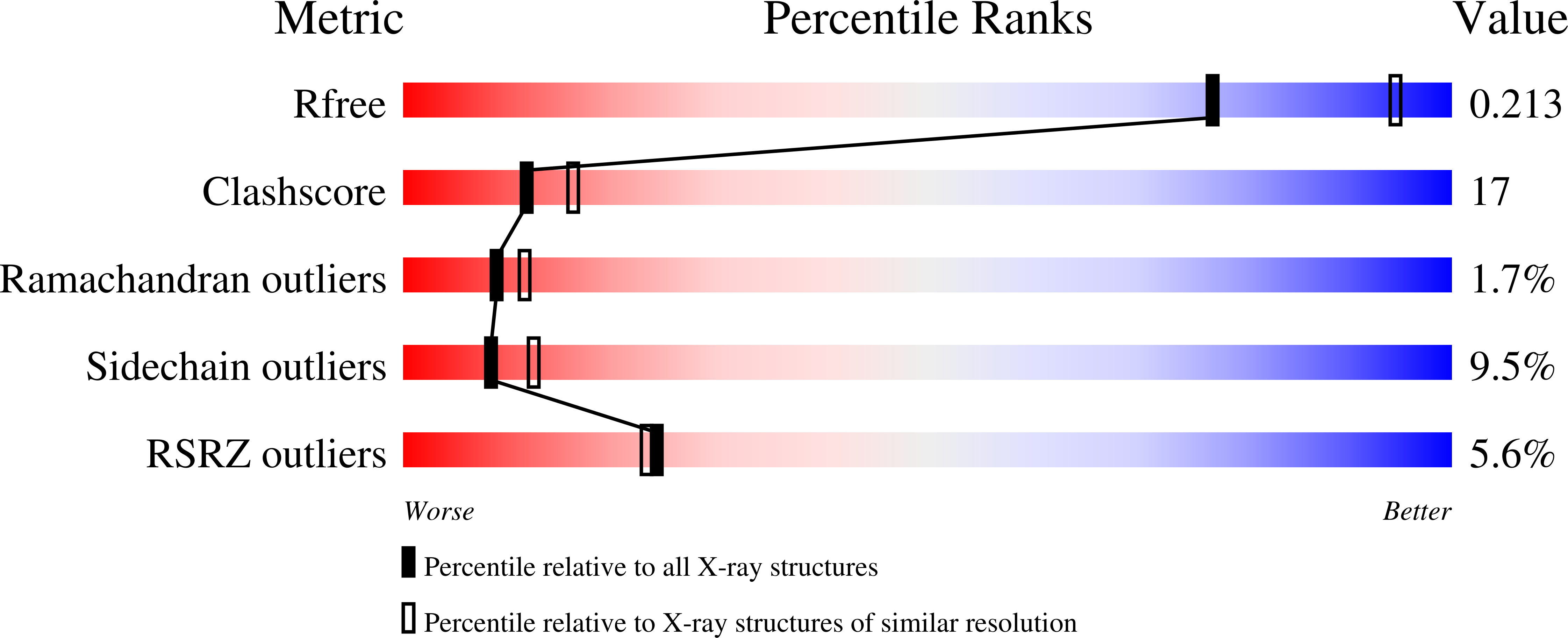
Structural Evidence of Substrate Specificity in Mammalian Peroxidases: STRUCTURE OF THE THIOCYANATE COMPLEX WITH LACTOPEROXIDASE AND ITS INTERACTIONS AT 2.4 A RESOLUTION
Sheikh, I.A., Singh, A.K., Singh, N., Sinha, M., Singh, S.B., Bhushan, A., Kaur, P., Srinivasan, A., Sharma, S., Singh, T.P.(2009) J Biol Chem 284: 14849-14856
- PubMed: 19339248
- DOI: https://doi.org/10.1074/jbc.M807644200
- Primary Citation of Related Structures:
3ERH, 3ERI, 3FAQ - PubMed Abstract:
The crystal structure of the complex of lactoperoxidase (LPO) with its physiological substrate thiocyanate (SCN(-)) has been determined at 2.4A resolution. It revealed that the SCN(-) ion is bound to LPO in the distal heme cavity. The observed orientation of the SCN(-) ion shows that the sulfur atom is closer to the heme iron than the nitrogen atom. The nitrogen atom of SCN(-) forms a hydrogen bond with a water (Wat) molecule at position 6'. This water molecule is stabilized by two hydrogen bonds with Gln(423) N(epsilon2) and Phe(422) oxygen. In contrast, the placement of the SCN(-) ion in the structure of myeloperoxidase (MPO) occurs with an opposite orientation, in which the nitrogen atom is closer to the heme iron than the sulfur atom. The site corresponding to the positions of Gln(423), Phe(422) oxygen, and Wat(6)' in LPO is occupied primarily by the side chain of Phe(407) in MPO due to an entirely different conformation of the loop corresponding to the segment Arg(418)-Phe(431) of LPO. This arrangement in MPO does not favor a similar orientation of the SCN(-) ion. The orientation of the catalytic product OSCN(-) as reported in the structure of LPO.OSCN(-) is similar to the orientation of SCN(-) in the structure of LPO.SCN(-). Similarly, in the structure of LPO.SCN(-).CN(-), in which CN(-) binds at Wat(1), the position and orientation of the SCN(-) ion are also identical to that observed in the structure of LPO.SCN.
Organizational Affiliation:
Department of Biophysics, All India Institute of Medical Sciences, New Delhi 110 029, India.