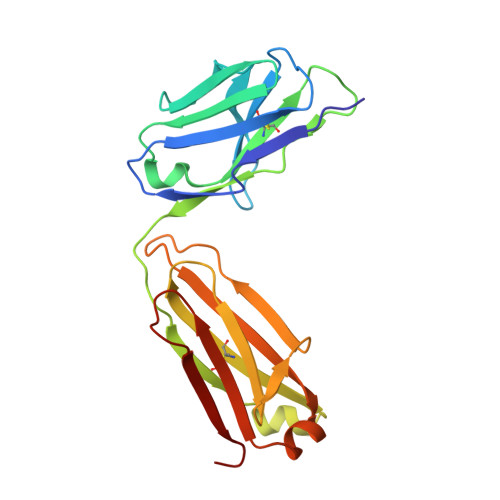
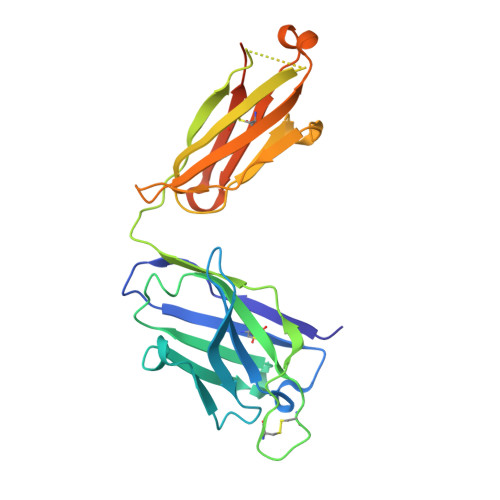
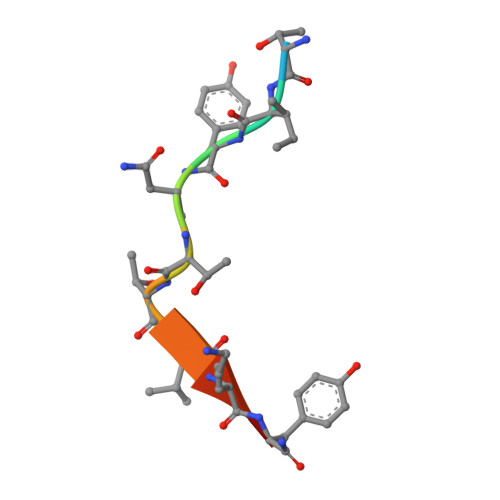
Germline V-genes sculpt the binding site of a family of antibodies neutralizing human cytomegalovirus.
Thomson, C.A., Bryson, S., McLean, G.R., Creagh, A.L., Pai, E.F., Schrader, J.W.(2008) EMBO J 27: 2592-2602
- PubMed: 18772881
- DOI: https://doi.org/10.1038/emboj.2008.179
- Primary Citation of Related Structures:
3EYF, 3EYO, 3EYQ, 3F12 - PubMed Abstract:
Immunoglobulin genes are generated somatically through specialized mechanisms resulting in a vast repertoire of antigen-binding sites. Despite the stochastic nature of these processes, the V-genes that encode most of the antigen-combining site are under positive evolutionary selection, raising the possibility that V-genes have been selected to encode key structural features of binding sites of protective antibodies against certain pathogens. Human, neutralizing antibodies to human cytomegalovirus that bind the AD-2S1 epitope on its gB envelope protein repeatedly use a pair of well-conserved, germline V-genes IGHV3-30 and IGKV3-11. Here, we present crystallographic, kinetic and thermodynamic analyses of the binding site of such an antibody and that of its primary immunoglobulin ancestor. These show that these germline V-genes encode key side chain contacts with the viral antigen and thereby dictate key structural features of the hypermutated, high-affinity neutralizing antibody. V-genes may thus encode an innate, protective immunological memory that targets vulnerable, invariant sites on multiple pathogens.
Organizational Affiliation:
The Biomedical Research Centre, University of British Columbia, Vancouver, British Columbia, Canada.