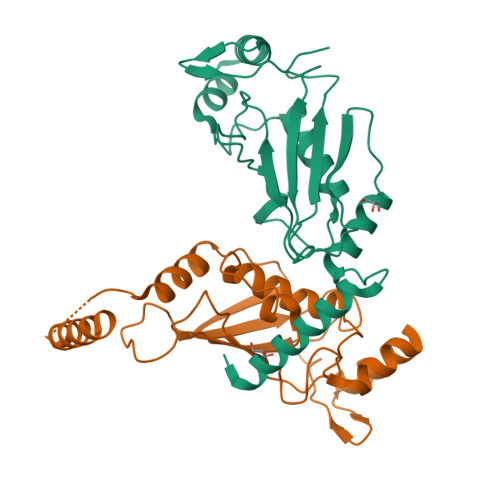
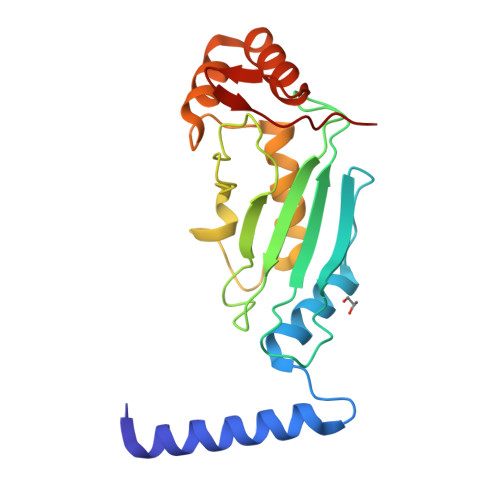
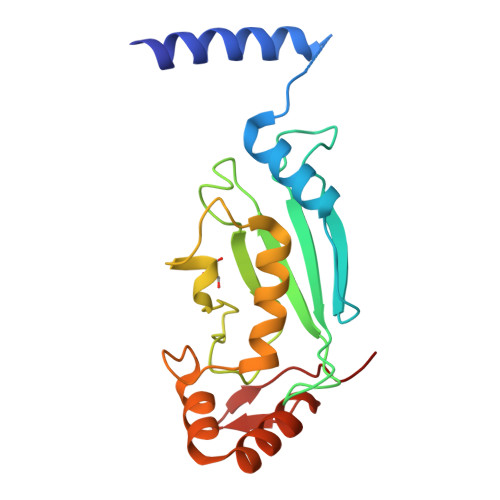
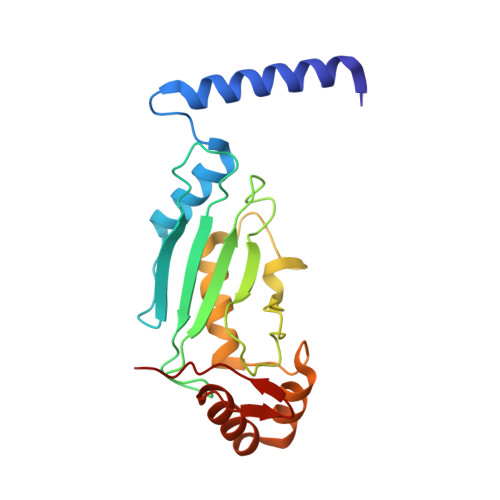
A dual E3 mechanism for Rub1 ligation to Cdc53.
Scott, D.C., Monda, J.K., Grace, C.R., Duda, D.M., Kriwacki, R.W., Kurz, T., Schulman, B.A.(2010) Mol Cell 39: 784-796
- PubMed: 20832729
- DOI: https://doi.org/10.1016/j.molcel.2010.08.030
- Primary Citation of Related Structures:
3O2P, 3O2U, 3O6B - PubMed Abstract:
In ubiquitin-like protein (UBL) cascades, a thioester-linked E2∼UBL complex typically interacts with an E3 enzyme for UBL transfer to the target. Here we demonstrate a variant mechanism, whereby the E2 Ubc12 functions with two E3s, Hrt1 and Dcn1, for ligation of the UBL Rub1 to Cdc53's WHB subdomain. Hrt1 functions like a conventional RING E3, with its N terminus recruiting Cdc53 and C-terminal RING activating Ubc12∼Rub1. Dcn1's "potentiating neddylation" domain (Dcn1(P)) acts as an additional E3, reducing nonspecific Hrt1-mediated Ubc12∼Rub1 discharge and directing Ubc12's active site to Cdc53. Crystal structures of Dcn1(P)-Cdc53(WHB) and Ubc12 allow modeling of a catalytic complex, supported by mutational data. We propose that Dcn1's interactions with both Cdc53 and Ubc12 would restrict the otherwise flexible Hrt1 RING-bound Ubc12∼Rub1 to a catalytically competent orientation. Our data reveal mechanisms by which two E3s function synergistically to promote UBL transfer from one E2 to a target.
Organizational Affiliation:
Howard Hughes Medical Institute, St Jude Children's Research Hospital, Memphis, TN 38105, USA.