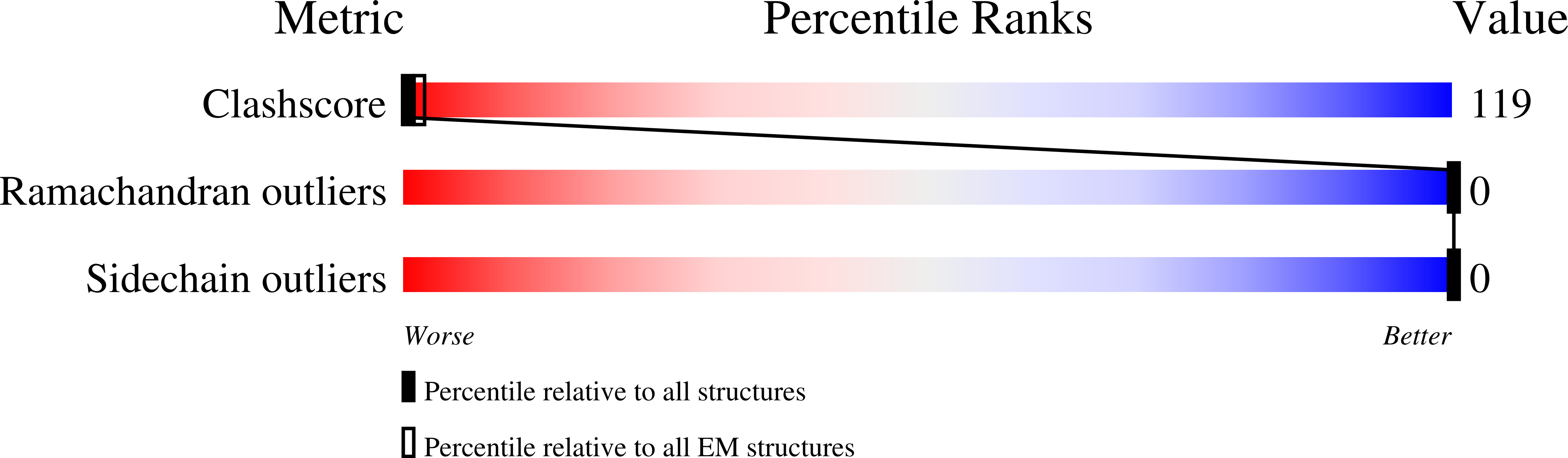Acetylcholine-Binding Protein in the Hemolymph of the Planorbid Snail Biomphalaria Glabrata is a Pentagonal Dodecahedron (60 Subunits)
Saur, M., Moeller, V., Kapetanopoulos, K., Braukmann, S., Gebauer, W., Tenzer, S., Markl, J.(2012) PLoS One 7: 43685
- PubMed: 22916297
- DOI: https://doi.org/10.1371/journal.pone.0043685
- Primary Citation of Related Structures:
4AOD, 4AOE - PubMed Abstract:
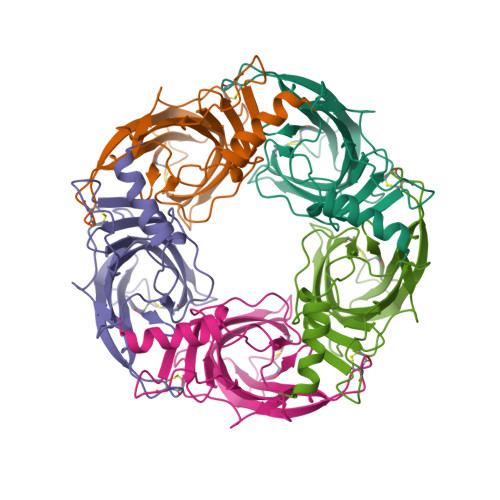
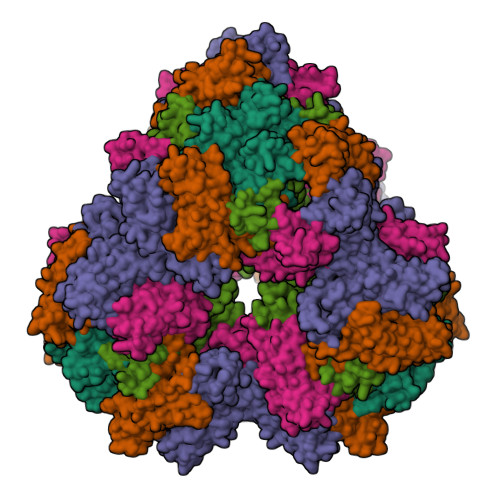
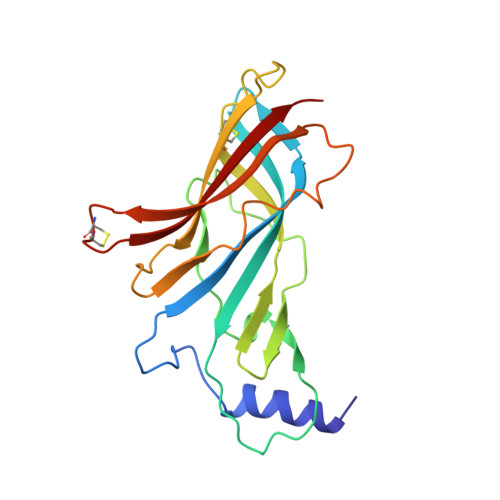
Nicotinic acetylcholine receptors (nAChR) play important neurophysiological roles and are of considerable medical relevance. They have been studied extensively, greatly facilitated by the gastropod acetylcholine-binding proteins (AChBP) which represent soluble structural and functional homologues of the ligand-binding domain of nAChR. All these proteins are ring-like pentamers. Here we report that AChBP exists in the hemolymph of the planorbid snail Biomphalaria glabrata (vector of the schistosomiasis parasite) as a regular pentagonal dodecahedron, 22 nm in diameter (12 pentamers, 60 active sites). We sequenced and recombinantly expressed two ∼25 kDa polypeptides (BgAChBP1 and BgAChBP2) with a specific active site, N-glycan site and disulfide bridge variation. We also provide the exon/intron structures. Recombinant BgAChBP1 formed pentamers and dodecahedra, recombinant BgAChBP2 formed pentamers and probably disulfide-bridged di-pentamers, but not dodecahedra. Three-dimensional electron cryo-microscopy (3D-EM) yielded a 3D reconstruction of the dodecahedron with a resolution of 6 Å. Homology models of the pentamers docked to the 6 Å structure revealed opportunities for chemical bonding at the inter-pentamer interfaces. Definition of the ligand-binding pocket and the gating C-loop in the 6 Å structure suggests that 3D-EM might lead to the identification of functional states in the BgAChBP dodecahedron.
Organizational Affiliation:
Institute of Zoology, Johannes Gutenberg University, Mainz, Germany.