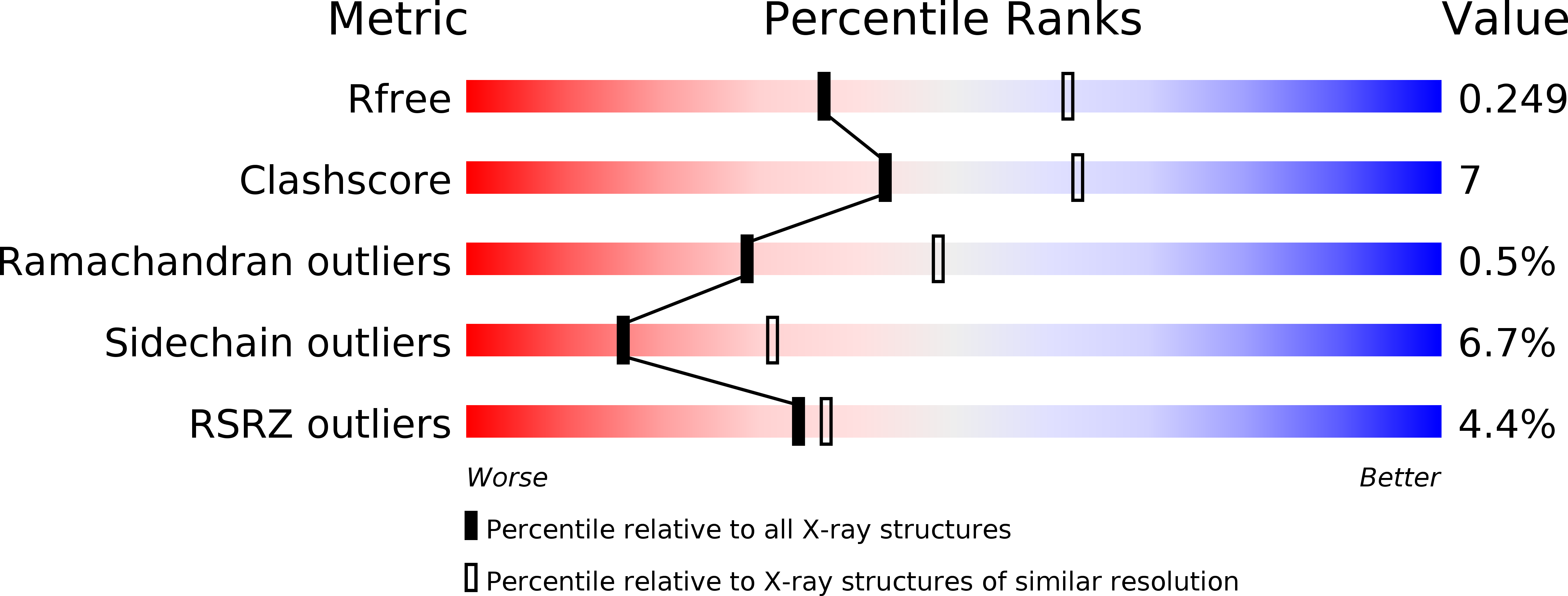
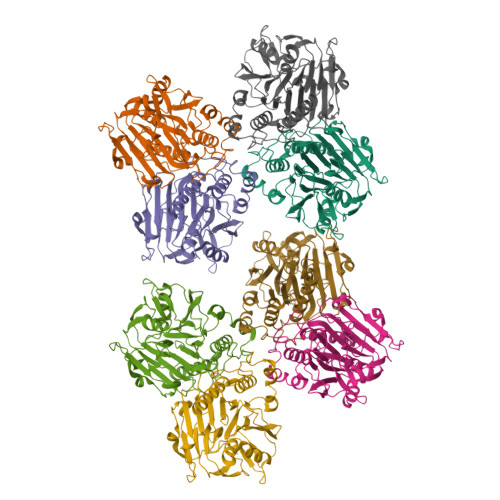
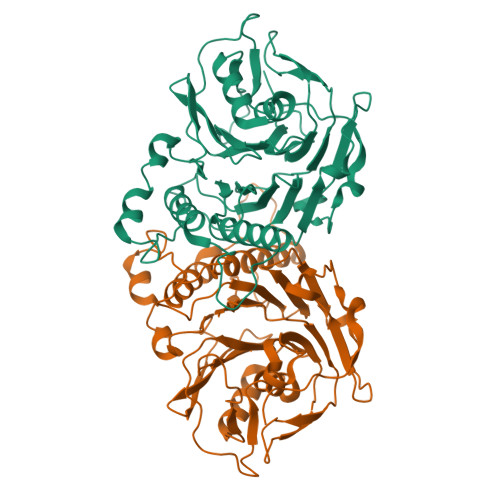
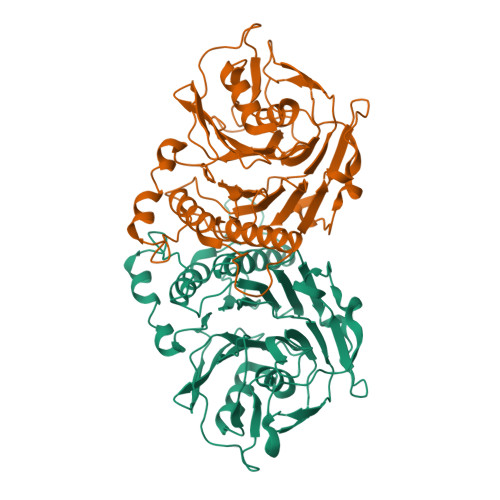
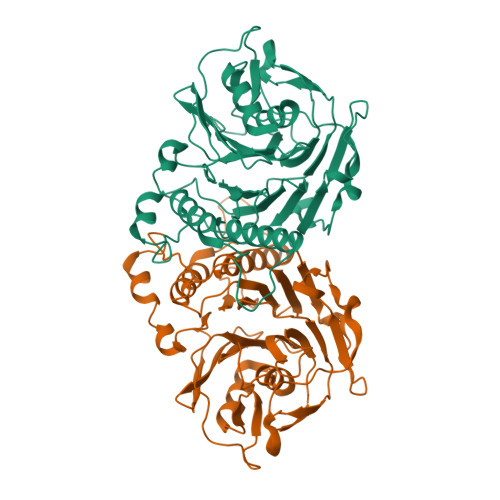
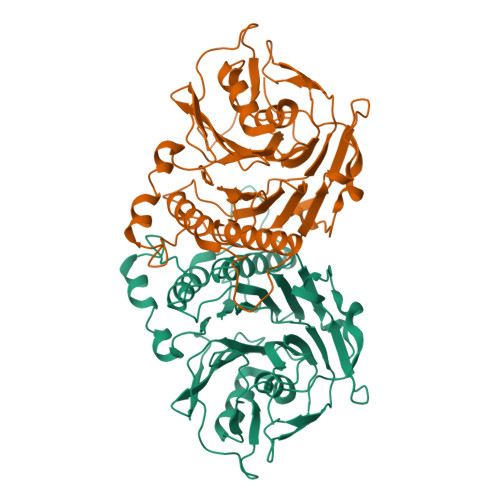
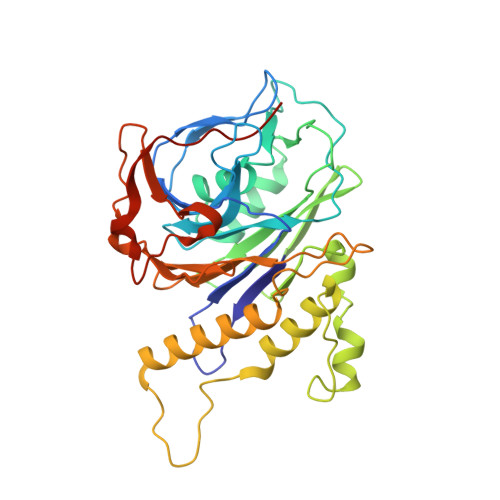
Structural analysis of a penicillin V acylase from Pectobacterium atrosepticum confirms the importance of two Trp residues for activity and specificity
Avinash, V.S., Panigrahi, P., Chand, D., Pundle, A., Suresh, C.G., Ramasamy, S.(2016) J Struct Biol 193: 85-94
- PubMed: 26707624
- DOI: https://doi.org/10.1016/j.jsb.2015.12.008
- Primary Citation of Related Structures:
4WL2 - PubMed Abstract:
Penicillin V acylases (PVA) catalyze the deacylation of the beta-lactam antibiotic phenoxymethylpenicillin (Pen V). They are members of the Ntn hydrolase family and possess an N-terminal cysteine as the main catalytic nucleophile residue. They form the evolutionarily related cholylglycine hydrolase (CGH) group which includes bile salt hydrolases (BSH) responsible for bile deconjugation. Even though a few PVA and BSH structures have been reported, no structure of a functional PVA from Gram-negative bacteria is available. Here, we report the crystal structure of a highly active PVA from Gram-negative Pectobacterium atrosepticum (PaPVA) at 2.5Å resolution. Structural comparison with PVAs from Gram-positive bacteria revealed that PaPVA had a distinctive tetrameric structure and active site organization. In addition, mutagenesis of key active site residues and biochemical characterization of the resultant variants elucidated the role of these residues in substrate binding and catalysis. The importance of residue Trp23 and Trp87 side chains in binding and correct positioning of Pen V by PVAs was confirmed using mutagenesis and substrate docking with a 15ns molecular dynamics simulation. These results establish the unique nature of Gram-negative CGHs and necessitate further research about their substrate spectrum.
Organizational Affiliation:
Biochemical Sciences Division, National Chemical Laboratory (CSIR-NCL), Pune 411008, India.