Kinetic consequences of re-engineering the outer shell "canopy" above the active site of a [NiFe]-hydrogenase.
Carr, S.B., Phillips, S.E.V., Evans, R.M., Brooke, E.J., Islam, S.T.A., Roberts, G.M., Wehlin, S.A.M., Armstrong, F.A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hydrogenase-1 small chain | A [auth S], C [auth T] | 335 | Escherichia coli CFT073 | Mutation(s): 0 EC: 1.12.99.6 | 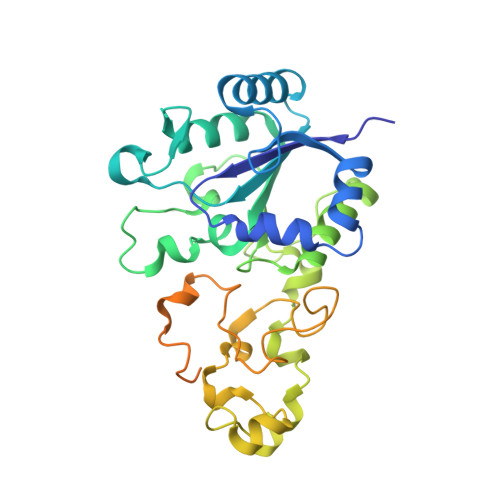 |
UniProt | |||||
Find proteins for P69739 (Escherichia coli (strain K12)) Explore P69739 Go to UniProtKB: P69739 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P69739 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Hydrogenase-1 large chain | B [auth L], D [auth M] | 582 | Escherichia coli K-12 | Mutation(s): 0 EC: 1.12.99.6 | 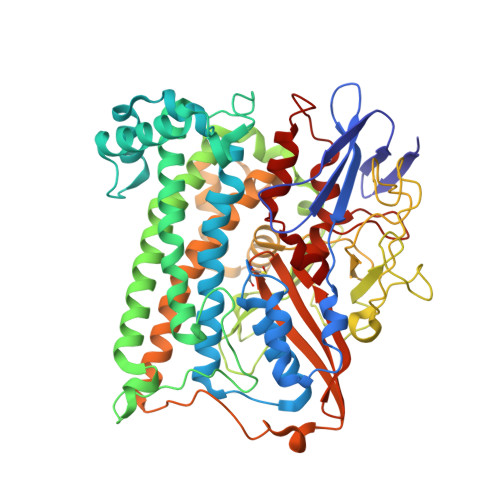 |
UniProt | |||||
Find proteins for P0ACD8 (Escherichia coli (strain K12)) Explore P0ACD8 Go to UniProtKB: P0ACD8 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0ACD8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 11 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| LMT Query on LMT | H [auth S], S [auth T] | DODECYL-BETA-D-MALTOSIDE C24 H46 O11 NLEBIOOXCVAHBD-QKMCSOCLSA-N |  | ||
| SF4 Query on SF4 | E [auth S], P [auth T] | IRON/SULFUR CLUSTER Fe4 S4 LJBDFODJNLIPKO-UHFFFAOYSA-N |  | ||
| SF3 Query on SF3 | G [auth S], R [auth T] | FE4-S3 CLUSTER Fe4 S3 QQACTBFBZNWJMV-UHFFFAOYSA-N |  | ||
| F3S Query on F3S | F [auth S], Q [auth T] | FE3-S4 CLUSTER Fe3 S4 FCXHZBQOKRZXKS-UHFFFAOYSA-N |  | ||
| FCO Query on FCO | L, W [auth M] | CARBONMONOXIDE-(DICYANO) IRON C3 Fe N2 O VBQUCMTXYFMTTE-UHFFFAOYSA-N |  | ||
| TRS Query on TRS | BA [auth M] | 2-AMINO-2-HYDROXYMETHYL-PROPANE-1,3-DIOL C4 H12 N O3 LENZDBCJOHFCAS-UHFFFAOYSA-O |  | ||
| SO4 Query on SO4 | J [auth S], K [auth L], V [auth T], Z [auth M] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| NI Query on NI | M [auth L], X [auth M] | NICKEL (II) ION Ni VEQPNABPJHWNSG-UHFFFAOYSA-N |  | ||
| CL Query on CL | I [auth S], T, U [auth T] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| MG Query on MG | N [auth L], Y [auth M] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| LI Query on LI | AA [auth M], O [auth L] | LITHIUM ION Li HBBGRARXTFLTSG-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSO Query on CSO | B [auth L], D [auth M] | L-PEPTIDE LINKING | C3 H7 N O3 S |  | CYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 93.981 | α = 90 |
| b = 97.799 | β = 90 |
| c = 183.474 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DIALS | data reduction |
| Aimless | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Biotechnology and Biological Sciences Research Council | United Kingdom | -- |