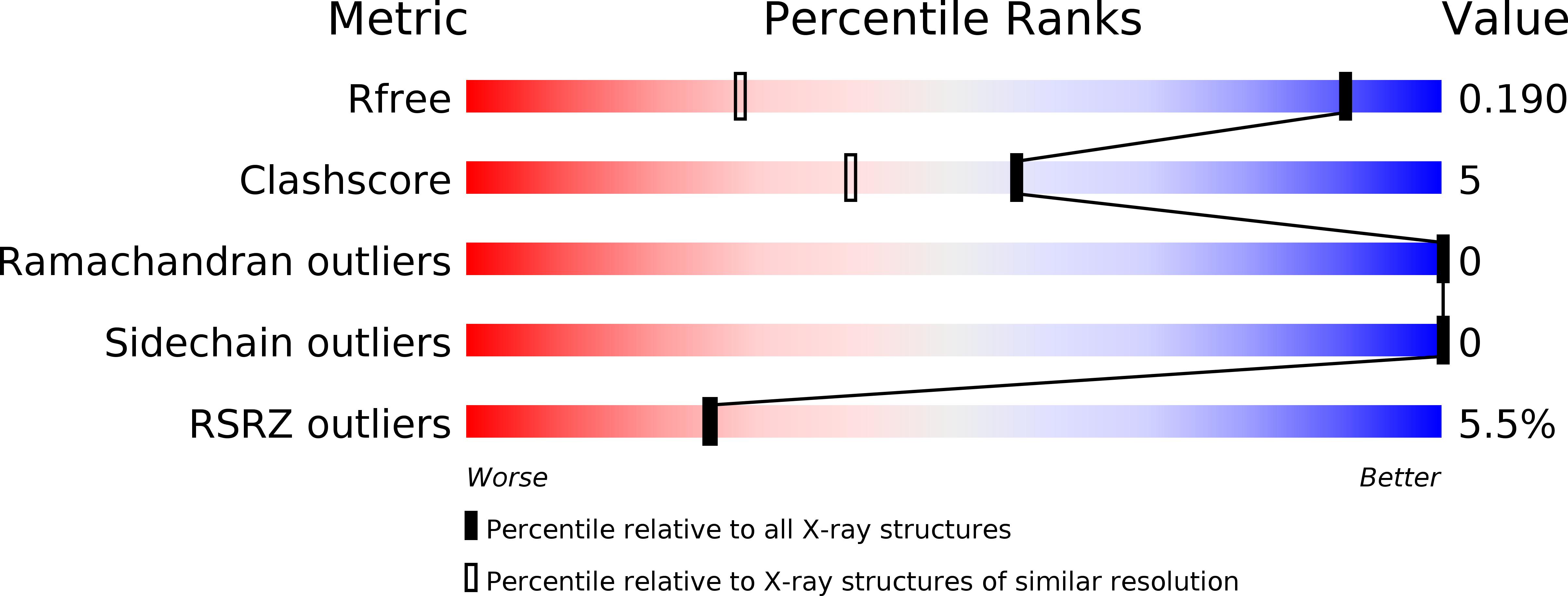
PanDDA analysis group deposition of models with modelled events (e.g. bound ligands)
Schuller, M., Talon, R., Krojer, T., Brandao-Neto, J., Douangamath, A., Zhang, R., von Delft, F., Schuler, H., Kessler, B., Knapp, S., Bountra, C., Arrowsmith, C.H., Edwards, A., Elkins, J.To be published.