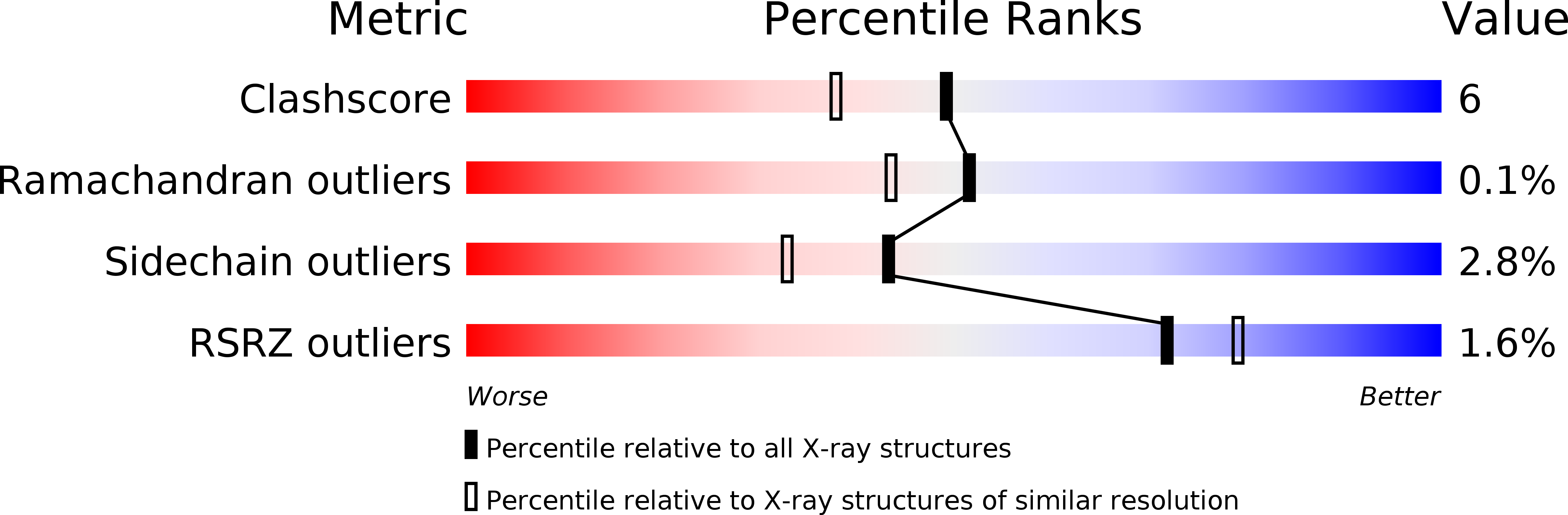
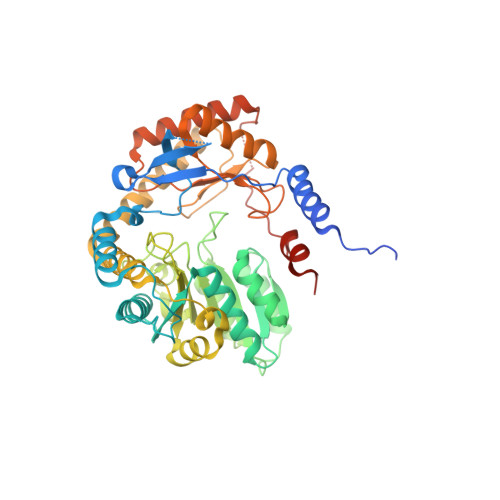
PanDDA analysis group deposition of ground-state model
Bezerra, G.A., Foster, W., Bailey, H., Shrestha, L., Krojer, T., Talon, R., Brandao-Neto, J., Douangamath, A., Nicola, B.B., von Delft, F., Arrowsmith, C.H., Edwards, A., Bountra, C., Brennan, P.E., Yue, W.W.To be published.