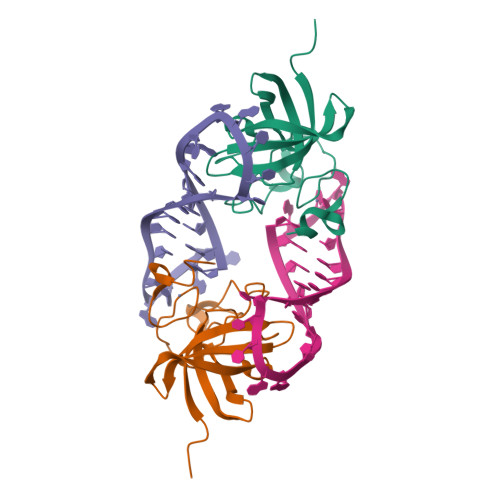
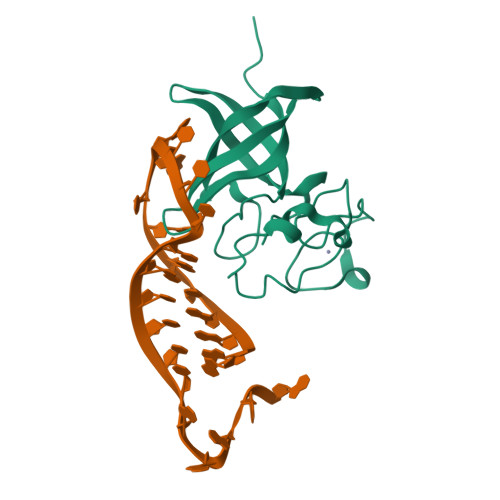
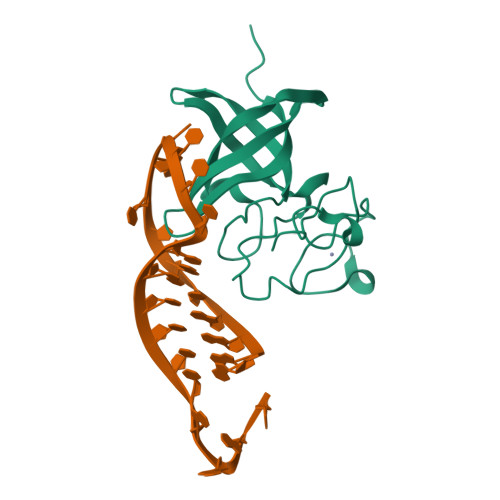
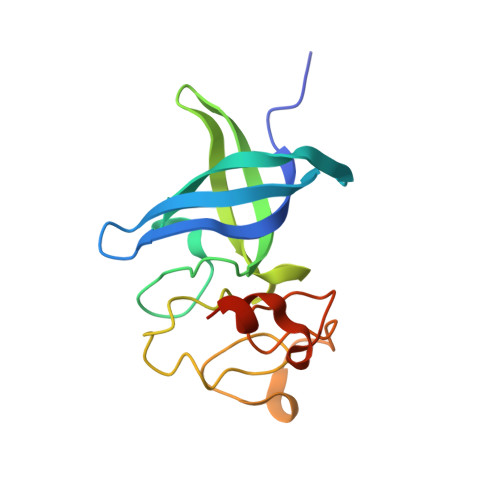
LIN28 Zinc Knuckle Domain Is Required and Sufficient to Induce let-7 Oligouridylation.
Wang, L., Nam, Y., Lee, A.K., Yu, C., Roth, K., Chen, C., Ransey, E.M., Sliz, P.(2017) Cell Rep 18: 2664-2675
- PubMed: 28297670
- DOI: https://doi.org/10.1016/j.celrep.2017.02.044
- Primary Citation of Related Structures:
5UDZ - PubMed Abstract:
LIN28 is an RNA binding protein that plays crucial roles in pluripotency, glucose metabolism, tissue regeneration, and tumorigenesis. LIN28 binds to the let-7 primary and precursor microRNAs through bipartite recognition and induces degradation of let-7 precursors (pre-let-7) by promoting oligouridylation by terminal uridylyltransferases (TUTases). Here, we report that the zinc knuckle domain (ZKD) of mouse LIN28 recruits TUT4 to initiate the oligouridylation of let-7 precursors. Our crystal structure of human LIN28 in complex with a fragment of pre-let-7f-1 determined to 2.0 Å resolution shows that the interaction between ZKD and RNA is constrained to a small cavity with a high druggability score. We demonstrate that the specific interaction between ZKD and pre-let-7 is necessary and sufficient to induce oligouridylation by recruiting the N-terminal fragment of TUT4 (NTUT4) and the formation of a stable ZKD:NTUT4:pre-let-7 ternary complex is crucial for the acquired processivity of TUT4.
Organizational Affiliation:
Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA.