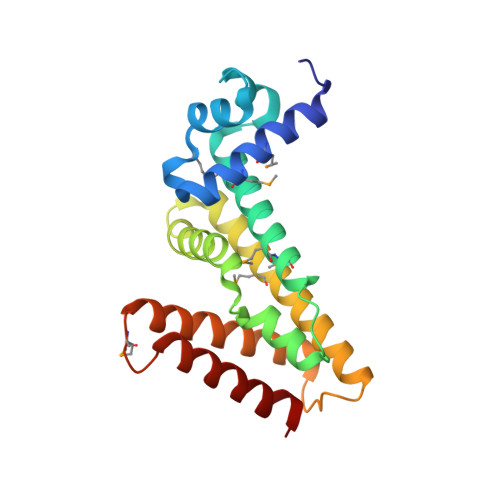
Jungle Express is a versatile repressor system for tight transcriptional control.
Ruegg, T.L., Pereira, J.H., Chen, J.C., DeGiovanni, A., Novichkov, P., Mutalik, V.K., Tomaleri, G.P., Singer, S.W., Hillson, N.J., Simmons, B.A., Adams, P.D., Thelen, M.P.(2018) Nat Commun 9: 3617-3617
- PubMed: 30190458
- DOI: https://doi.org/10.1038/s41467-018-05857-3
- Primary Citation of Related Structures:
5VL9, 5VLG, 5VLM - PubMed Abstract:
Tightly regulated promoters are essential for numerous biological applications, where strong inducibility, portability, and scalability are desirable. Current systems are often incompatible with large-scale fermentations due to high inducer costs and strict media requirements. Here, we describe the bottom-up engineering of 'Jungle Express', an expression system that enables efficient gene regulation in diverse proteobacteria. This system is guided by EilR, a multidrug-binding repressor with high affinity to its optimized operator and cationic dyes that act as powerful inducers at negligible costs. In E. coli, the engineered promoters exhibit minimal basal transcription and are inducible over four orders of magnitude by 1 µM crystal violet, reaching expression levels exceeding those of the strongest current bacterial systems. Further, we provide molecular insights into specific interactions of EilR with its operator and with two inducers. The versatility of Jungle Express opens the way for tightly controlled and efficient gene expression that is not restricted to host organism, substrate, or scale.
Organizational Affiliation:
Joint BioEnergy Institute, Emeryville, CA, 94608, USA.