Structures of the Heme Acquisition Protein HasA with Iron(III)-5,15-Diphenylporphyrin and Derivatives Thereof as an Artificial Prosthetic Group
Uehara, H., Shisaka, Y., Nishimura, T., Sugimoto, H., Shiro, Y., Miyake, Y., Shinokubo, H., Watanabe, Y., Shoji, O.(2017) Angew Chem Int Ed Engl 56: 15279-15283
- PubMed: 28921809
- DOI: https://doi.org/10.1002/anie.201707212
- Primary Citation of Related Structures:
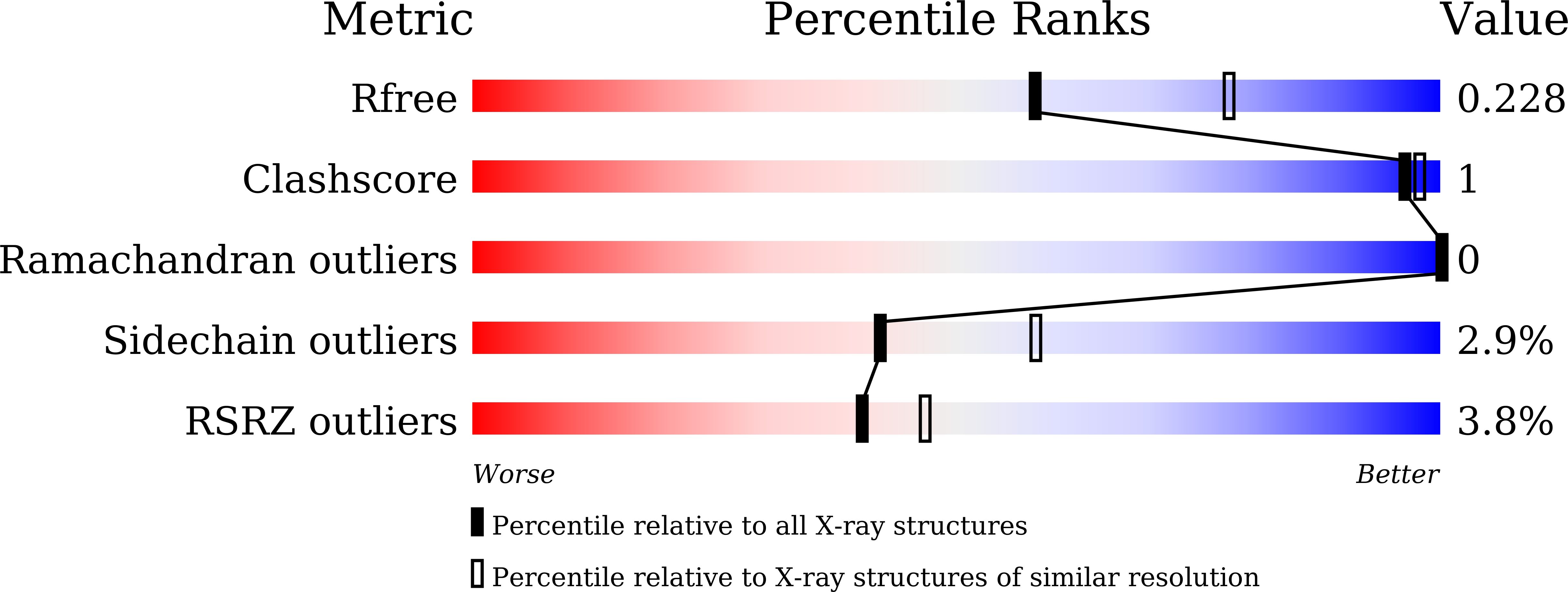
5XA4, 5XIB, 5XIC, 5XIE, 5XKB - PubMed Abstract:
Iron(III)-5,15-diphenylporphyrin and several derivatives were accommodated by HasA, a heme acquisition protein secreted by Pseudomonas aeruginosa, despite possessing bulky substituents at the meso position of the porphyrin. Crystal structure analysis revealed that the two phenyl groups at the meso positions of porphyrin extend outside HasA. It was shown that the growth of P. aeruginosa was inhibited in the presence of HasA coordinating the synthetic porphyrins under iron-limiting conditions, and that the structure of the synthetic porphyrins greatly affects the inhibition efficiency.
Organizational Affiliation:
Department of Chemistry, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8602, Japan.