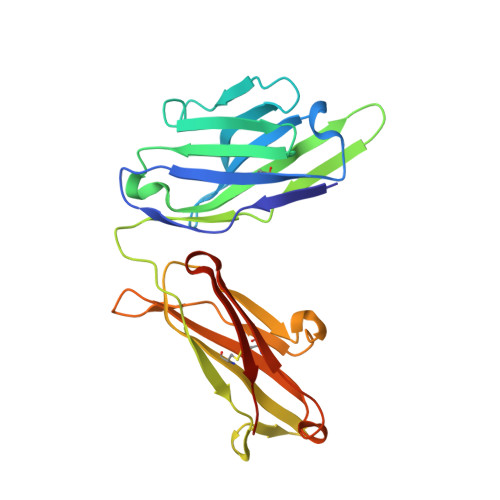
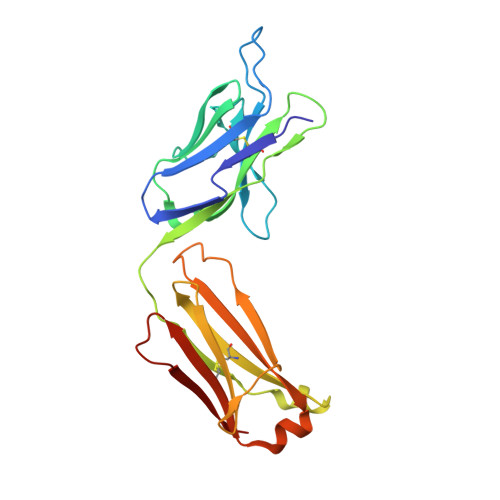
A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite.
Kisalu, N.K., Idris, A.H., Weidle, C., Flores-Garcia, Y., Flynn, B.J., Sack, B.K., Murphy, S., Schon, A., Freire, E., Francica, J.R., Miller, A.B., Gregory, J., March, S., Liao, H.X., Haynes, B.F., Wiehe, K., Trama, A.M., Saunders, K.O., Gladden, M.A., Monroe, A., Bonsignori, M., Kanekiyo, M., Wheatley, A.K., McDermott, A.B., Farney, S.K., Chuang, G.Y., Zhang, B., Kc, N., Chakravarty, S., Kwong, P.D., Sinnis, P., Bhatia, S.N., Kappe, S.H.I., Sim, B.K.L., Hoffman, S.L., Zavala, F., Pancera, M., Seder, R.A.(2018) Nat Med 24: 408-416
- PubMed: 29554083
- DOI: https://doi.org/10.1038/nm.4512
- Primary Citation of Related Structures:
6B5L, 6B5M, 6B5N, 6B5O, 6B5P, 6B5R, 6B5S, 6B5T - PubMed Abstract:
Development of a highly effective vaccine or antibodies for the prevention and ultimately elimination of malaria is urgently needed. Here we report the isolation of a number of human monoclonal antibodies directed against the Plasmodium falciparum (Pf) circumsporozoite protein (PfCSP) from several subjects immunized with an attenuated Pf whole-sporozoite (SPZ) vaccine (Sanaria PfSPZ Vaccine). Passive transfer of one of these antibodies, monoclonal antibody CIS43, conferred high-level, sterile protection in two different mouse models of malaria infection. The affinity and stoichiometry of CIS43 binding to PfCSP indicate that there are two sequential multivalent binding events encompassing the repeat domain. The first binding event is to a unique 'junctional' epitope positioned between the N terminus and the central repeat domain of PfCSP. Moreover, CIS43 prevented proteolytic cleavage of PfCSP on PfSPZ. Analysis of crystal structures of the CIS43 antigen-binding fragment in complex with the junctional epitope determined the molecular interactions of binding, revealed the epitope's conformational flexibility and defined Asn-Pro-Asn (NPN) as the structural repeat motif. The demonstration that CIS43 is highly effective for passive prevention of malaria has potential application for use in travelers, military personnel and elimination campaigns and identifies a new and conserved site of vulnerability on PfCSP for next-generation rational vaccine design.
Organizational Affiliation:
Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.