Substrate preference of an ABC importer corresponds to selective growth on beta-(1,6)-galactosides inBifidobacterium animalissubsp.lactis.
Theilmann, M.C., Fredslund, F., Svensson, B., Lo Leggio, L., Abou Hachem, M.(2019) J Biol Chem 294: 11701-11711
- PubMed: 31186348
- DOI: https://doi.org/10.1074/jbc.RA119.008843
- Primary Citation of Related Structures:
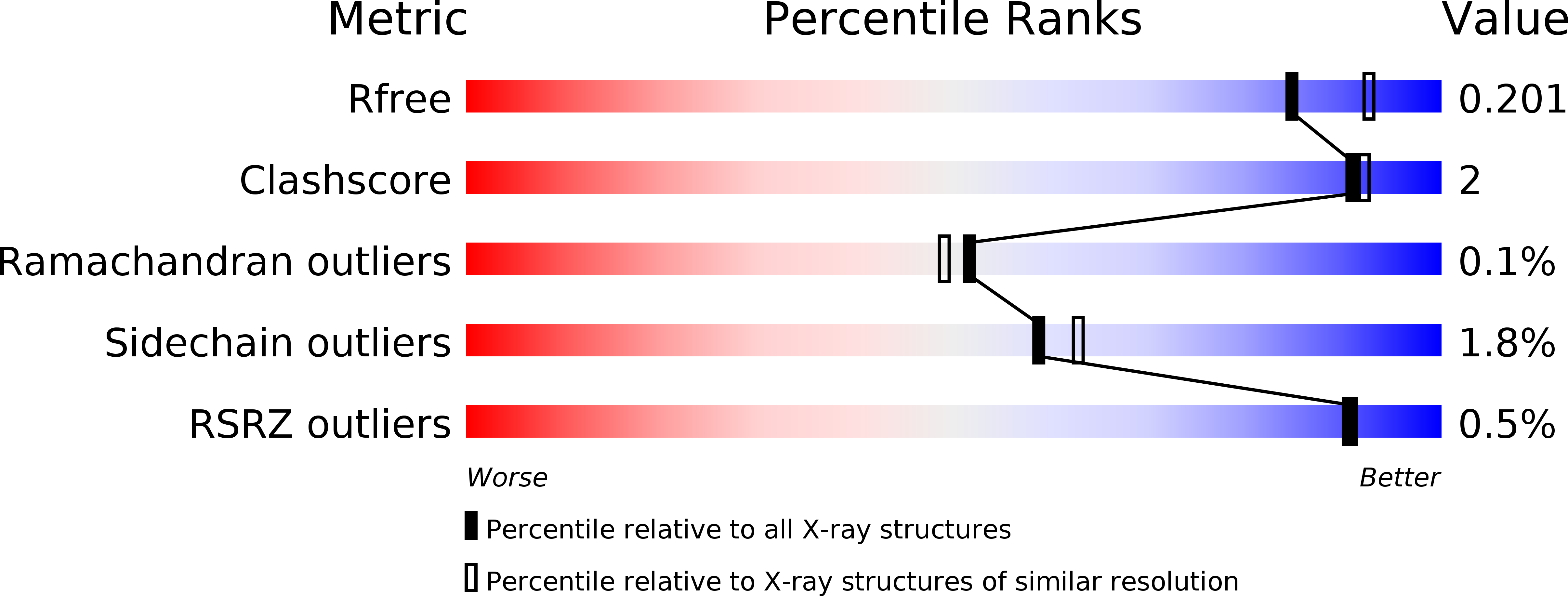
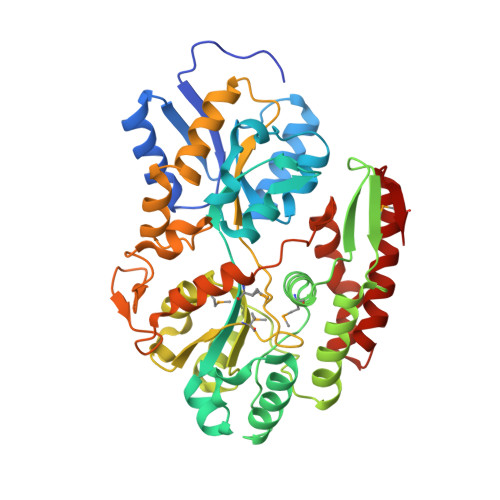
6H0H, 6Q5G - PubMed Abstract:
Bifidobacteria are exposed to substantial amounts of dietary β-galactosides. Distinctive preferences for growth on different β-galactosides are observed within Bifidobacterium members, but the basis of these preferences remains unclear. We previously described the first β-(1,6)/(1,3)-galactosidase from Bifidobacterium animalis subsp. lactis Bl-04. This enzyme is relatively promiscuous, exhibiting only 5-fold higher efficiency on the preferred β-(1,6)-galactobiose than the β-(1,4) isomer. Here, we characterize the solute-binding protein ( Bal 6GBP) that governs the specificity of the ABC transporter encoded by the same β-galactoside utilization locus. We observed that although Bal 6GBP recognizes both β-(1,6)- and β-(1,4)-galactobiose, Bal 6GBP has a 1630-fold higher selectivity for the former, reflected in dramatic differences in growth, with several hours lag on less preferred β-(1,4)- and β-(1,3)-galactobiose. Experiments performed in the presence of varying proportions of β-(1,4)/β-(1,6)-galactobioses indicated that the preferred substrate was preferentially depleted from the culture supernatant. This established that the poor growth on the nonpreferred β-(1,4) was due to inefficient uptake. We solved the structure of Bal 6GBP in complex with β-(1,6)-galactobiose at 1.39 Å resolution, revealing the structural basis of this strict selectivity. Moreover, we observed a close evolutionary relationship with the human milk disaccharide lacto- N -biose-binding protein from Bifidobacterium longum , indicating that the recognition of the nonreducing galactosyl is essentially conserved, whereas the adjacent position is diversified to fit different glycosidic linkages and monosaccharide residues. These findings indicate that oligosaccharide uptake has a pivotal role in governing selectivity for distinct growth substrates and have uncovered evolutionary trajectories that shape the diversification of sugar uptake proteins within Bifidobacterium .
Organizational Affiliation:
Department of Biotechnology and Biomedicine, Technical University of Denmark, Søltofts Plads, Building 224, DK-2800 Kgs. Lyngby, Denmark.