Targeting BRD4 and PI3K signaling pathways for the treatment of medulloblastoma.
Sethi, B., Kumar, V., Jayasinghe, T.D., Dong, Y., Ronning, D.R., Zhong, H.A., Coulter, D.W., Mahato, R.I.(2023) J Control Release 354: 80-90
- PubMed: 36599397
- DOI: https://doi.org/10.1016/j.jconrel.2022.12.055
- Primary Citation of Related Structures:
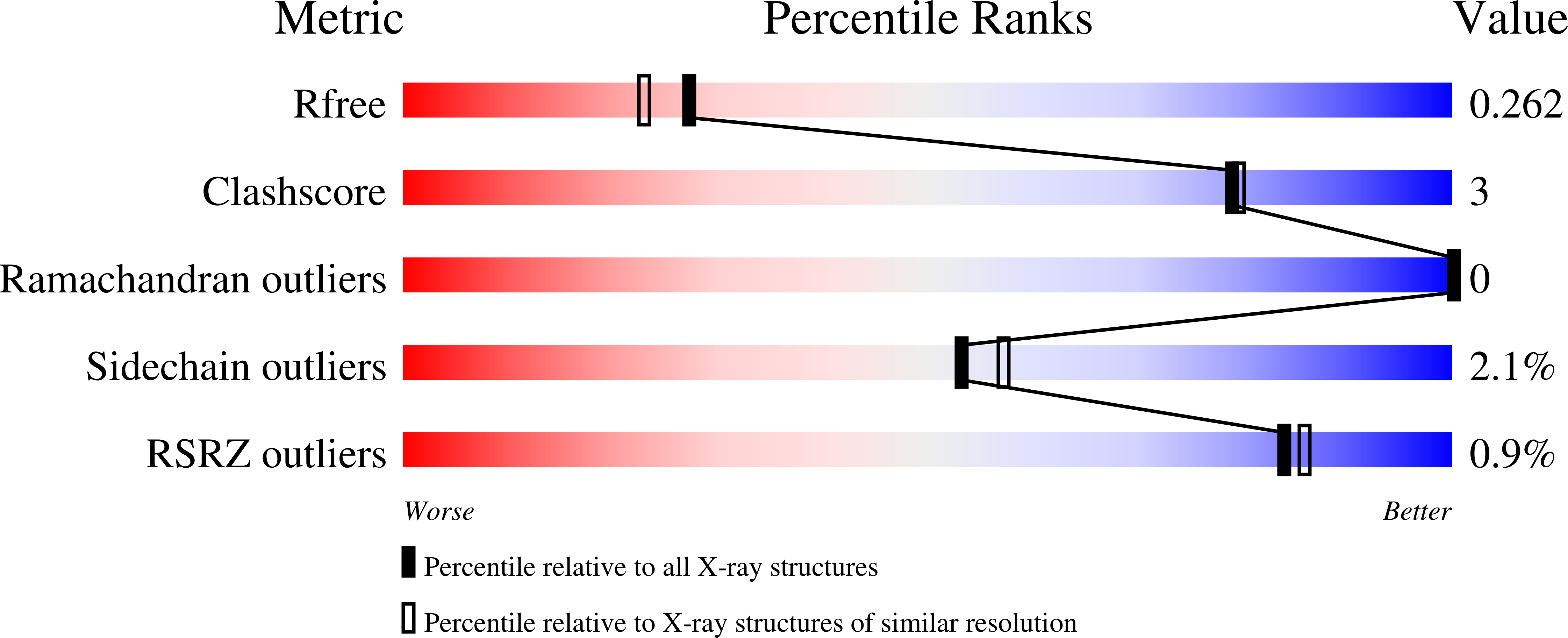
7USG, 7USH, 7USI, 7USJ, 7USK - PubMed Abstract:
Medulloblastoma (MB) is a malignant pediatric brain tumor which shows upregulation of MYC and sonic hedgehog (SHH) signaling. SHH inhibitors face acquired resistance, which is a major cause of relapse. Further, direct MYC oncogene inhibition is challenging, inhibition of MYC upstream insulin-like growth factor/ phosphatidylinositol-4,5-bisphosphate 3-kinase (IGF/PI3K) is a promising alternative. While PI3K inhibition activates resistance mechanisms, simultaneous inhibition of bromodomain-containing protein 4 (BRD4) and PI3K can overcome resistance. We synthesized a new molecule 8-(2,3-dihydrobenzo[b] [1, 4] dioxin-6-yl)-2-morpholino-4H-chromen-4-one (MDP5) that targets both BRD4 and PI3K pathways. We used X-ray crystal structures and a molecular modeling approach to confirm the interactions between MDP5 with bromo domains (BDs) from both BRD2 and BRD4, and molecular modeling for PI3K binding. MDP5 was shown to inhibit target pathways and MB cell growth in vitro and in vivo. MDP5 showed higher potency in DAOY cells (IC 50 5.5 μM) compared to SF2523 (IC 50 12.6 μM), and its IC 50 values in HD-MB03 cells were like SF2523. Treatment of MB cells with MDP5 significantly decreased colony formation, increased apoptosis, and halted cell cycle progression. Further, MDP5 was well tolerated in NSG mice bearing either xenograft or orthotopic MB tumors at the dose of 20 mg/kg, and significantly reduced tumor growth and prolonged animal survival.
Organizational Affiliation:
Department of Pharmaceutical Sciences, the University of Nebraska Medical Center, Omaha, NE 68198, USA.