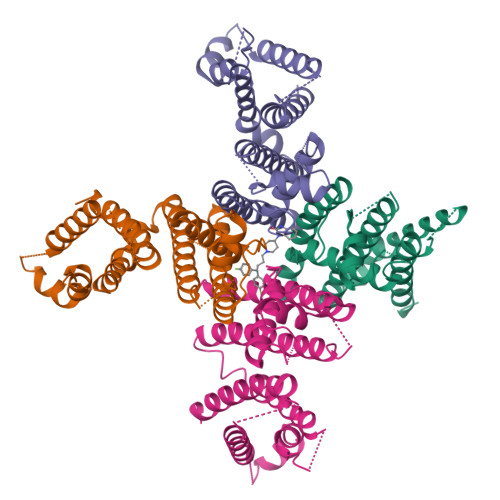
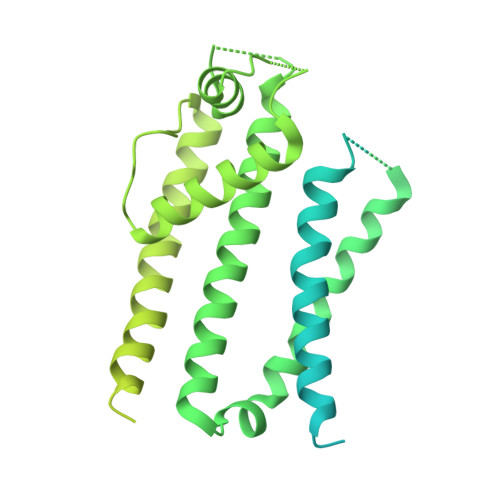
Improved higher resolution cryo-EM structures reveal the binding modes of hERG channel inhibitors.
Miyashita, Y., Moriya, T., Kato, T., Kawasaki, M., Yasuda, S., Adachi, N., Suzuki, K., Ogasawara, S., Saito, T., Senda, T., Murata, T.(2024) Structure 32: 1926
- PubMed: 39321803
- DOI: https://doi.org/10.1016/j.str.2024.08.021
- Primary Citation of Related Structures:
8ZYN, 8ZYO, 8ZYP, 8ZYQ - PubMed Abstract:
During drug discovery, it is crucial to exclude compounds with toxic effects. The human ether-à-go-go-related gene (hERG) channel is essential for maintaining cardiac repolarization and is a critical target in drug safety evaluation due to its role in drug-induced arrhythmias. Inhibition of the hERG channel can lead to severe cardiac issues, including Torsades de Pointes tachycardia. Understanding hERG inhibition mechanisms is essential to avoid these toxicities. Several structural studies have elucidated the interactions between inhibitors and hERG. However, orientation and resolution issues have so far limited detailed insights. Here, we used digitonin to analyze the apo state of hERG, which resolved orientation issues and improved the resolution. We determined the structure of hERG bound to astemizole, showing a clear map in the pore pathway. Using this strategy, we also analyzed the binding modes of E-4031 and pimozide. These insights into inhibitor interactions with hERG may aid safer drug design and enhance cardiac safety.
Organizational Affiliation:
Department of Developmental Biology, Graduate School of Medicine, Chiba University, 1-8-1 Inohana, Chuo, Chiba 260-8670, Japan; Department of Chemistry, Graduate School of Science, Chiba University, 1-33 Yayoi-cho, Inage, Chiba 263-8522, Japan.