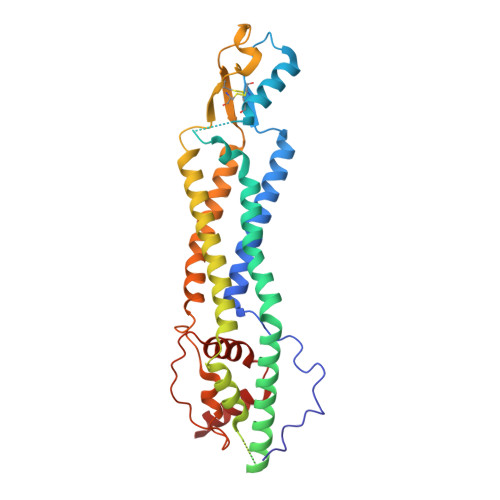
The C-terminal activating domain promotes pannexin 1 channel opening.
Henze, E., Ehrlich, J.J., Robertson, J.L., Gelsleichter, E., Kawate, T.(2024) Proc Natl Acad Sci U S A 121: e2411898121-e2411898121
- PubMed: 39671183
- DOI: https://doi.org/10.1073/pnas.2411898121
- Primary Citation of Related Structures:
9BZ7, 9BZ8 - PubMed Abstract:
Pannexin 1 (Panx1) constitutes a large pore channel responsible for the release of adenosine triphosphate (ATP) from apoptotic cells. Strong evidence indicates that caspase-mediated cleavage of the C-terminus promotes the opening of the Panx1 channel by unplugging the pore. However, this simple pore-plugging mechanism alone cannot account for the observation that a Panx1 construct ending before the caspase cleavage site remains closed. Here, we show that a helical region located immediately before the caspase cleavage site, referred to as the "C-terminal activating domain (CAD)", plays a pivotal role in facilitating Panx1 activation. Electrophysiology and mutagenesis studies uncovered that two conserved leucine residues within the CAD play a pivotal role. Cryoelectron microscopy (Cryo-EM) analysis of the construct ending before reaching the CAD demonstrated that the N terminus extends into an intracellular pocket. In contrast, the construct including the CAD revealed that this domain occupies the intracellular pocket, causing the N terminus to flip upward within the pore. Analysis of electrostatic free energy landscape in the closed conformation indicated that the intracellular side of the ion permeation pore may be occupied by anions like ATP, creating an electrostatic barrier for anions attempting to permeate the pore. When the N terminus flips up, it diminishes the positively charged surface, thereby reducing the drive to accumulate anions inside the pore. This dynamic change in the electrostatic landscape likely contributes to the selection of permeant ions. Collectively, these experiments put forth a mechanism in which C-terminal cleavage liberates the CAD, causing the repositioning of the N terminus to promote Panx1 channel opening.
Organizational Affiliation:
Department of Molecular Medicine, Cornell University, Ithaca, NY 14853.