More than an Amide Bioisostere: Discovery of 1,2,4-Triazole-containing Pyrazolo[1,5- a ]pyrimidine Host CSNK2 Inhibitors for Combatting beta-Coronavirus Replication.
Ong, H.W., Yang, X., Smith, J.L., Dickmander, R.J., Brown, J.W., Havener, T.M., Taft-Benz, S., Howell, S., Sanders, M.K., Capener, J.L., Counago, R.M., Chang, E., Kramer, A., Moorman, N.J., Heise, M., Axtman, A.D., Drewry, D.H., Willson, T.M.(2024) J Med Chem 67: 12261-12313
- PubMed: 38959455
- DOI: https://doi.org/10.1021/acs.jmedchem.4c00962
- Primary Citation of Related Structures:
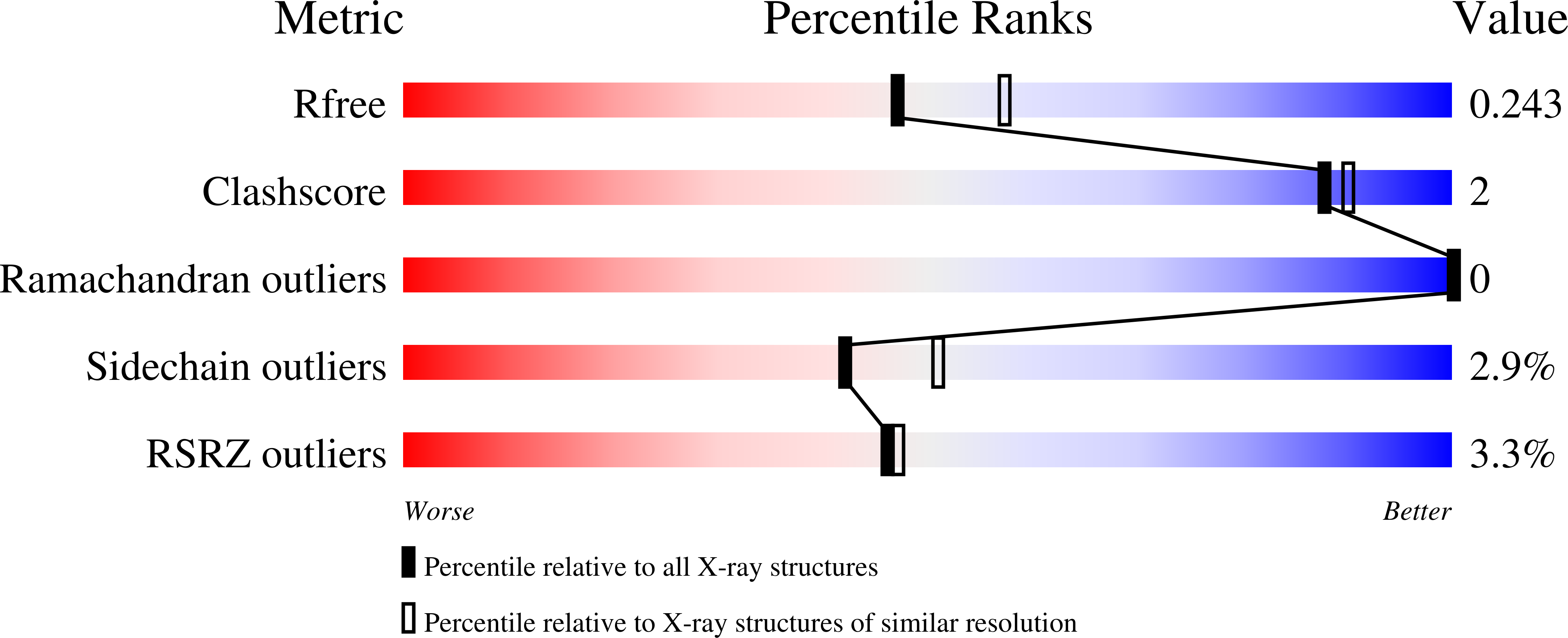
8P06, 8P07, 9EZG - PubMed Abstract:
The pyrazolo[1,5- a ]pyrimidine scaffold is a promising scaffold to develop potent and selective CSNK2 inhibitors with antiviral activity against β-coronaviruses. Herein, we describe the discovery of a 1,2,4-triazole group to substitute a key amide group for CSNK2 binding present in many potent pyrazolo[1,5- a ]pyrimidine inhibitors. Crystallographic evidence demonstrates that the 1,2,4-triazole replaces the amide in forming key hydrogen bonds with Lys68 and a water molecule buried in the ATP-binding pocket. This isosteric replacement improves potency and metabolic stability at a cost of solubility. Optimization for potency, solubility, and metabolic stability led to the discovery of the potent and selective CSNK2 inhibitor 53 . Despite excellent in vitro metabolic stability, rapid decline in plasma concentration of 53 in vivo was observed and may be attributed to lung accumulation, although in vivo pharmacological effect was not observed. Further optimization of this novel chemotype may validate CSNK2 as an antiviral target in vivo.
Organizational Affiliation:
Rapidly Emerging Antiviral Drug Development Initiative (READDI), Chapel Hill, North Carolina 27599, United States.