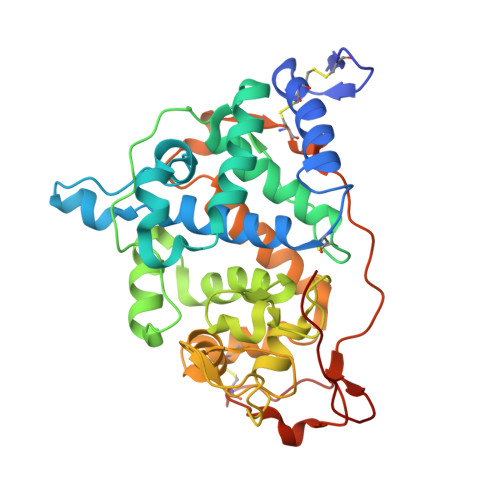
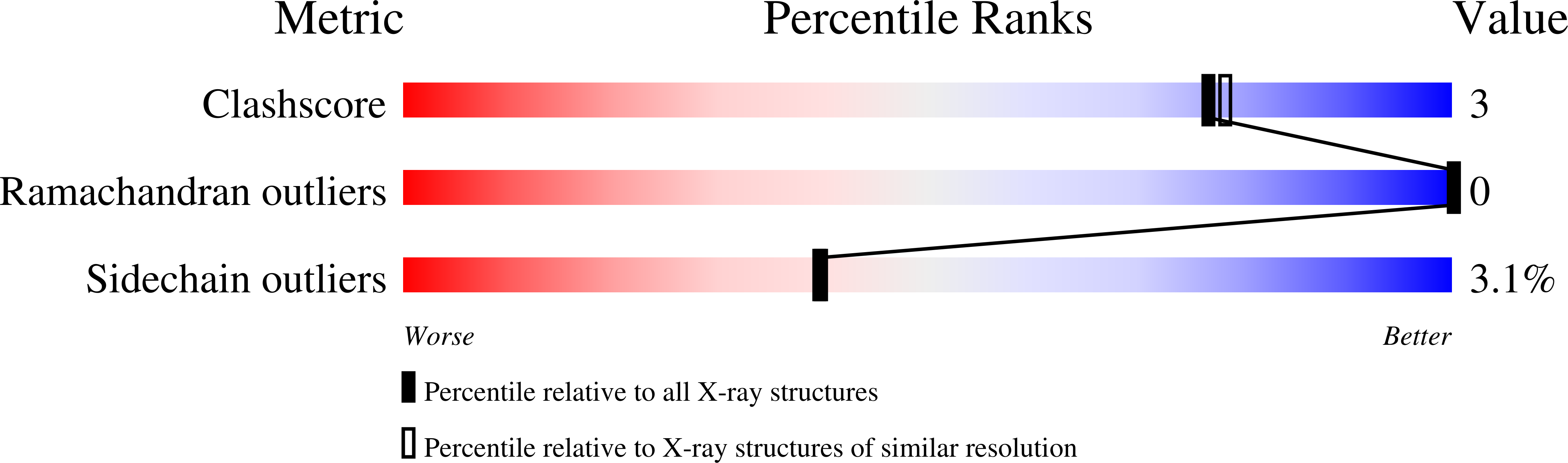Impact of the Physical and Chemical Environment on the Molecular Structure of Coprinus Cinereus Peroxidase
Houborg, K., Harris, P., Petersen, J.F.W., Rowland, P., Poulsen, J., Schneider, P., Vind, J., Larsen, S.(2003) Acta Crystallogr D Biol Crystallogr 59: 989
- PubMed: 12777760
- DOI: https://doi.org/10.1107/s0907444903006772
- Primary Citation of Related Structures:
1H3J, 1LY9, 1LYC, 1LYK - PubMed Abstract:
The structure of the peroxidase from Coprinus cinereus (CiP) has been determined in three different space groups and crystalline environments. Two of these are of the recombinant glycosylated form (rCiP), which crystallized in space groups P2(1)2(1)2(1) and C2. The third crystal form was obtained from a variant of CiP in which the glycosylation sites have been removed (rCiPON). It crystallizes in space group P2(1) with beta approximately 90 degrees; the structure was determined from room-temperature data and low-temperature data obtained from twinned crystals. Two independent molecules of CiP related by non-crystallographic symmetry are contained in the three crystal forms. The packing in the two structures of the glycosylated form of rCiP is closely related, but differs from the packing in the unglycosylated rCiPON. A database search based on small-molecule porphinato iron (III) complexes has been performed and related to observations of the spin states and coordination numbers of the iron ion. The room-temperature structures of CiP and one structure of the almost identical peroxidase from Arthromyces ramosus (ARP) have been used to identify 66 conserved water molecules and to assign a structural role to most of them.
Organizational Affiliation:
Centre for Crystallographic Studies, Department of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen, Denmark.