Snapshots of catalysis: the structure of fructose-1,6-(bis)phosphate aldolase covalently bound to the substrate dihydroxyacetone phosphate.
Choi, K.H., Shi, J., Hopkins, C.E., Tolan, D.R., Allen, K.N.(2001) Biochemistry 40: 13868-13875
- PubMed: 11705376
- DOI: https://doi.org/10.1021/bi0114877
- Primary Citation of Related Structures:
1J4E - PubMed Abstract:
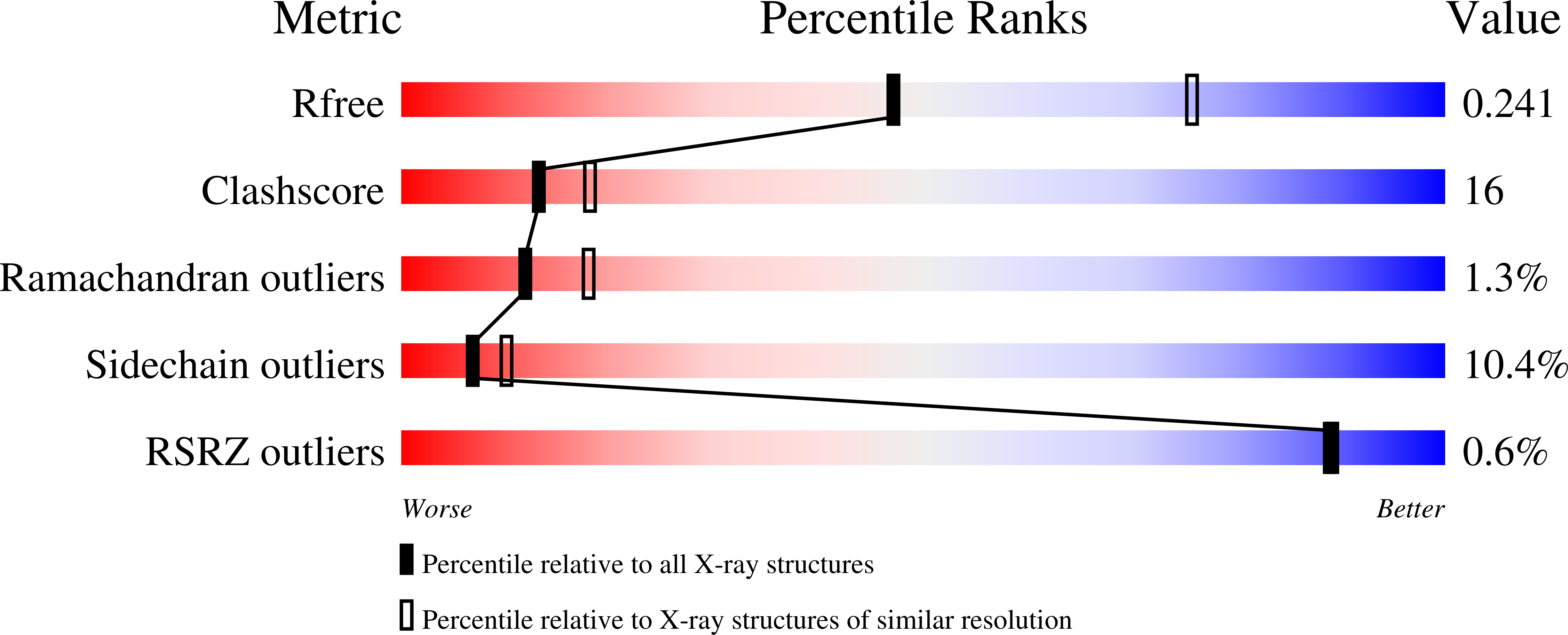
Fructose-1,6-bis(phosphate) aldolase is an essential glycolytic enzyme found in all vertebrates and higher plants that catalyzes the cleavage of fructose 1,6-bis(phosphate) (Fru-1,6-P(2)) to glyceraldehyde 3-phosphate and dihydroxyacetone phosphate (DHAP). Mutations in the aldolase genes in humans cause hemolytic anemia and hereditary fructose intolerance. The structure of the aldolase-DHAP Schiff base has been determined by X-ray crystallography to 2.6 A resolution (R(cryst) = 0.213, R(free) = 0.249) by trapping the catalytic intermediate with NaBH(4) in the presence of Fru-1,6-P(2). This is the first structure of a trapped covalent intermediate for this essential glycolytic enzyme. The structure allows the elucidation of a comprehensive catalytic mechanism and identification of a conserved chemical motif in Schiff-base aldolases. The position of the bound DHAP relative to Asp33 is consistent with a role for Asp33 in deprotonation of the C4-hydroxyl leading to C-C bond cleavage. The methyl side chain of Ala31 is positioned directly opposite the C3-hydroxyl, sterically favoring the S-configuration of the substrate at this carbon. The "trigger" residue Arg303, which binds the substrate C6-phosphate group, is a ligand to the phosphate group of DHAP. The observed movement of the ligand between substrate and product phosphates may provide a structural link between the substrate cleavage and the conformational change in the C-terminus associated with product release. The position of Glu187 in relation to the DHAP Schiff base is consistent with a role for the residue in protonation of the hydroxyl group of the carbinolamine in the dehydration step, catalyzing Schiff-base formation. The overlay of the aldolase-DHAP structure with that of the covalent enzyme-dihydroxyacetone structure of the mechanistically similar transaldolase and KDPG aldolase allows the identification of a conserved Lys-Glu dyad involved in Schiff-base formation and breakdown. The overlay highlights the fact that Lys146 in aldolase is replaced in transaldolase with Asn35. The substitution in transaldolase stabilizes the enamine intermediate required for the attack of the second aldose substrate, changing the chemistry from aldolase to transaldolase.
Organizational Affiliation:
Department of Biology, Boston University, 5 Cummington Street, Boston, Massachusetts 02215, USA.