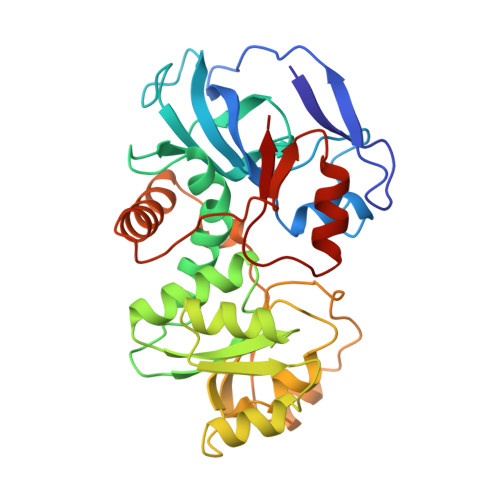
Crystal structure of Escherichia coli QOR quinone oxidoreductase complexed with NADPH.
Thorn, J.M., Barton, J.D., Dixon, N.E., Ollis, D.L., Edwards, K.J.(1995) J Mol Biology 249: 785-799
- PubMed: 7602590
- DOI: https://doi.org/10.1006/jmbi.1995.0337
- Primary Citation of Related Structures:
1QOR - PubMed Abstract:
The crystal structure of the homodimer of quinone oxidoreductase from Escherichia coli has been determined using the multiple isomorphous replacement method at 2.2 A resolution and refined to an R-factor of 14.1% The crystallographic asymmetric unit contains one functional dimer with the two subunits being related by a non-crystallographic 2-fold symmetry axis. The model consists of two polypeptide chains (residues 2 through 327), one NADPH molecule and one sulphate anion per subunit, and 432 water molecules. Each subunit consists of two domains: a catalytic domain and a nucleotide-binding domain with the NADPH co-factor bound in the cleft between domains. Quinone oxidoreductase has an unusual nucleotide-binding fingerprint motif consisting of the sequence AXXGXXG. The overall structure of quinone oxidoreductase shows strong structural homology to that of horse liver alcohol dehydrogenase.
- Centre for Molecular Structure and Function, Research School of Chemistry, Australian National University, Canberra.
Organizational Affiliation: