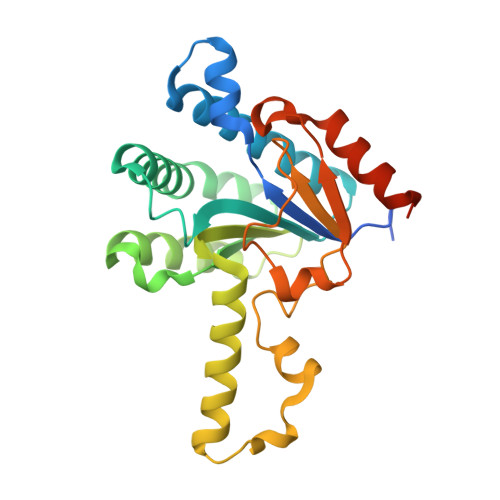
Substrate binding mode and reaction mechanism of undecaprenyl pyrophosphate synthase deduced from crystallographic studies
Chang, S.-Y., Ko, T.-P., Chen, A.P.-C., Wang, A.H.-J., Liang, P.-H.(2004) Protein Sci 13: 971-978
- PubMed: 15044730
- DOI: https://doi.org/10.1110/ps.03519904
- Primary Citation of Related Structures:
1V7U - PubMed Abstract:
Undecaprenyl pyrophosphate synthase (UPPs) catalyzes eight consecutive condensation reactions of farnesyl pyrophosphate (FPP) with isopentenyl pyrophosphate (IPP) to form a 55-carbon long-chain product. We previously reported the crystal structure of the apo-enzyme from Escherichia coli and the structure of UPPs in complex with sulfate ions (resembling pyrophosphate of substrate), Mg(2+), and two Triton molecules (product-like). In the present study, FPP substrate was soaked into the UPPs crystals, and the complex structure was solved. Based on the crystal structure, the pyrophosphate head group of FPP is bound to the backbone NHs of Gly29 and Arg30 as well as the side chains of Asn28, Arg30, and Arg39 through hydrogen bonds. His43 is close to the C2 carbon of FPP and may stabilize the farnesyl cation intermediate during catalysis. The hydrocarbon moiety of FPP is bound with hydrophobic amino acids including Leu85, Leu88, and Phe89, located on the alpha3 helix. The binding mode of FPP in cis-type UPPs is apparently different from that of trans-type and many other prenyltransferases which utilize Asprich motifs for substrate binding via Mg(2+). The new structure provides a plausible mechanism for the catalysis of UPPs.
- Institute of Biological Chemistry, Academia Sinica, Taipei 115, Taiwan.
Organizational Affiliation: