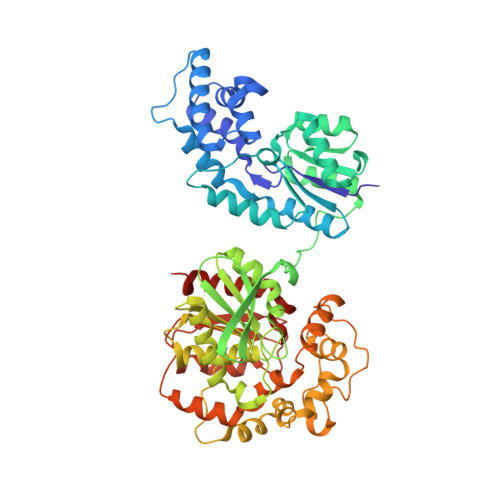
Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis
Gomez, G.A., Morisseau, C., Hammock, B.D., Christianson, D.W.(2004) Biochemistry 43: 4716-4723
- PubMed: 15096040
- DOI: https://doi.org/10.1021/bi036189j
- Primary Citation of Related Structures:
1S8O, 1VJ5 - PubMed Abstract:
The X-ray crystal structure of human soluble epoxide hydrolase (sEH) has been determined at 2.6 A resolution, revealing a domain-swapped quaternary structure identical to that observed for the murine enzyme [Argiriadi, M. A., Morisseau, C., Hammock, B. D., and Christianson, D. W. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10637-10642]. As with the murine enzyme, the epoxide hydrolytic mechanism of the human enzyme proceeds through an alkyl-enzyme intermediate with Asp-333 in the C-terminal domain. The structure of the human sEH complex with N-cyclohexyl-N'-(iodophenyl)urea (CIU) has been determined at 2.35 A resolution. Tyr-381 and Tyr-465 donate hydrogen bonds to the alkylurea carbonyl group of CIU, consistent with the proposed roles of these residues as proton donors in the first step of catalysis. The N-terminal domain of mammalian sEH contains a 15 A deep cleft, but its biological function is unclear. Recent experiments demonstrate that the N-terminal domain of human sEH catalyzes the metal-dependent hydrolysis of phosphate esters [Cronin, A., Mowbray, S., Dürk, H., Homburg, S., Fleming, I., Fisslthaler, B., Oesch, F., and Arand, M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1552-1557; Newman, J. W., Morisseau, C., Harris, T. R., and Hammock, B. D. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1558-1563]. The binding of Mg(2+)-HPO4(2-) to the N-terminal domain of human sEH in its CIU complex reveals structural features relevant to those of the enzyme-substrate complex in the phosphatase reaction.
- Roy and Diana Vagelos Laboratories, Department of Chemistry, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6323, USA.
Organizational Affiliation: