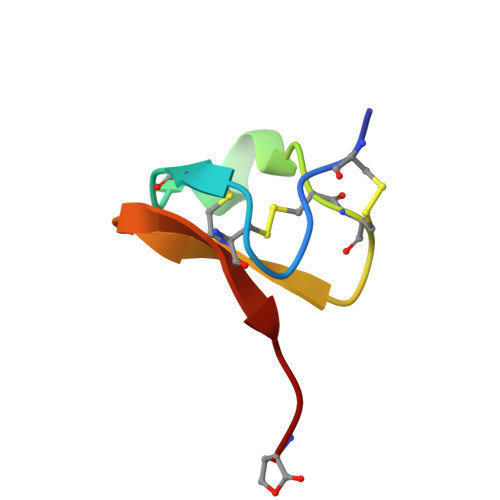
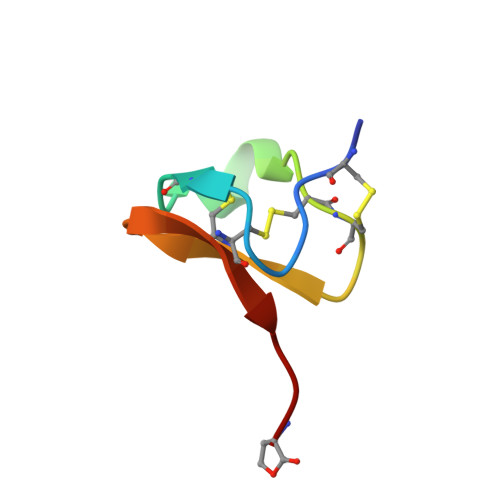
Solution structure of PcFK1, a spider peptide active against Plasmodium falciparum
Pimentel, C., Choi, S.J., Chagot, B., Guette, C., Camadro, J.M., Darbon, H.(2006) Protein Sci 15: 628-634
- PubMed: 16452619
- DOI: https://doi.org/10.1110/ps.051860606
- Primary Citation of Related Structures:
1X5V - PubMed Abstract:
Psalmopeotoxin I (PcFK1) is a 33-amino-acid residue peptide isolated from the venom of the tarantula Psalmopoeus cambridgei. It has been recently shown to possess strong antiplasmodial activity against the intra-erythrocyte stage of Plasmodium falciparum in vitro. Although the molecular target for PcFK1 is not yet determined, this peptide does not lyse erythrocytes, is not cytotoxic to nucleated mammalian cells, and does not inhibit neuromuscular function. We investigated the structural properties of PcFK1 to help understand the unique mechanism of action of this peptide and to enhance its utility as a lead compound for rational development of new antimalarial drugs. In this paper, we have determined the three-dimensional solution structure by (1)H two-dimensional NMR means of recombinant PcFK1, which is shown to belong to the ICK structural superfamily with structural determinants common to several neurotoxins acting as ion channels effectors.
Organizational Affiliation:
AFMB, CNRS UMR 6098 and Universités d'Aix-Marseille I and II, 31 Chemin Joseph Aiguier, 13402 Marseille Cedex 20, France.