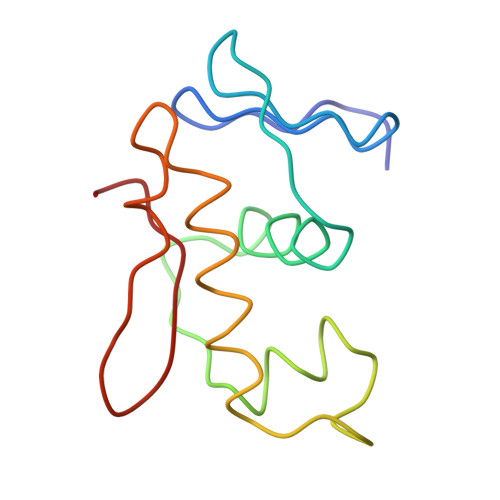
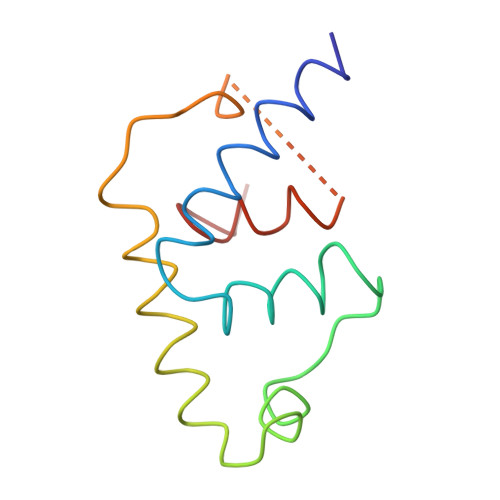
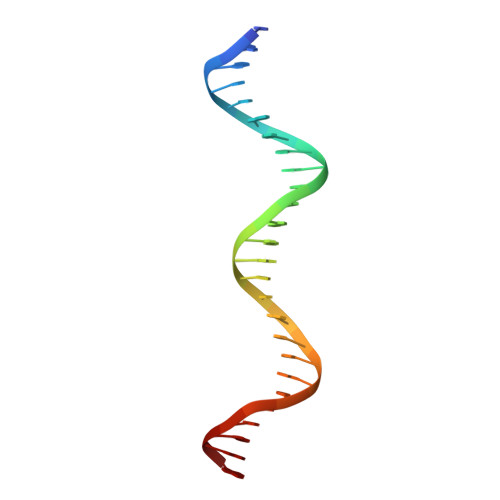
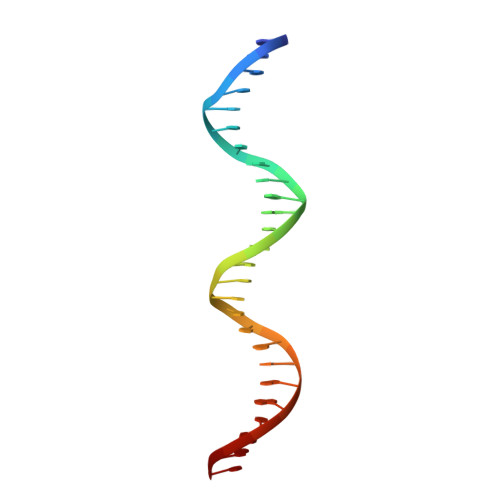
The structure of a transcription activation subcomplex reveals how sigma (70) is recruited to PhoB promoters.
Blanco, A.G., Canals, A., Bernues, J., Sola, M., Coll, M.(2011) EMBO J 30: 3776-3785
- PubMed: 21829166
- DOI: https://doi.org/10.1038/emboj.2011.271
- Primary Citation of Related Structures:
3T72 - PubMed Abstract:
PhoB is a two-component response regulator that activates transcription by interacting with the σ(70) subunit of the E. coli RNA polymerase in promoters in which the -35 σ(70)-recognition element is replaced by the pho box. The crystal structure of a transcription initiation subcomplex that includes the σ(4) domain of σ(70) fused with the RNA polymerase β subunit flap tip helix, the PhoB effector domain and the pho box DNA reveals how σ(4) recognizes the upstream pho box repeat. As with the -35 element, σ(4) achieves this recognition through the N-terminal portion of its DNA recognition helix, but contact with the DNA major groove is less extensive. Unexpectedly, the same recognition helix contacts the transactivation loop and helices α2 and α3 of PhoB. This result shows a simple and elegant mechanism for polymerase recruitment to pho box promoters in which the lost -35 element contacts are compensated by new ones with the activator. In addition, σ(4) is reoriented, thereby suggesting a remodelling mechanism for transcription initiation.
- Institute for Research in Biomedicine, Barcelona Science Park, Spain.
Organizational Affiliation: