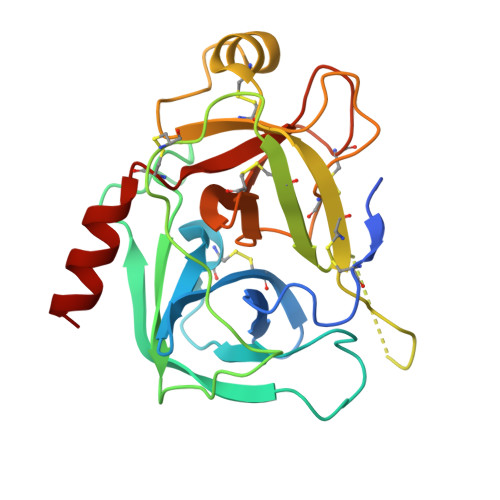
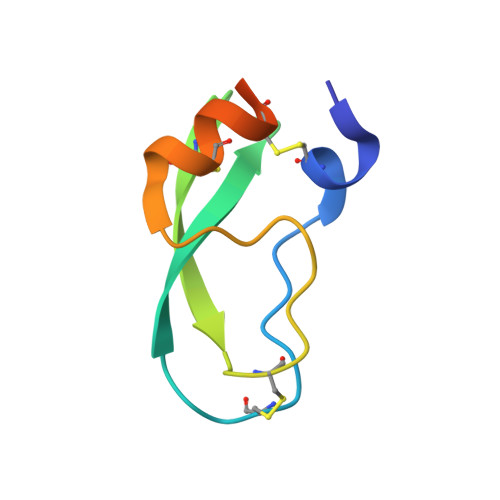
The energetic cost of induced fit catalysis: Crystal structures of trypsinogen mutants with enhanced activity and inhibitor affinity.
Pasternak, A., White, A., Jeffery, C.J., Medina, N., Cahoon, M., Ringe, D., Hedstrom, L.(2001) Protein Sci 10: 1331-1342
- PubMed: 11420435
- DOI: https://doi.org/10.1110/ps.44101
- Primary Citation of Related Structures:
1F5R, 1F7Z, 1FY8, 3TGK - PubMed Abstract:
The contribution of induced fit to enzyme specificity has been much debated, although with little experimental data. Here we probe the effect of induced fit on enzyme specificity using the trypsin(ogen) system. BPTI is known to induce trypsinogen to assume a trypsinlike conformation. Correlations are observed between BPTI affinity and the values of k(cat)/K(m) for the hydrolysis of two substrates by eight trypsin(ogen) variants. The slope of both correlations is -1.8. The crystal structures of the BPTI complexes of four variant trypsinogens were also solved. Three of these enzymes, K15A, DeltaI16V17/D194N, and DeltaI16V17/Q156K trypsinogen, are 10- to 100-fold more active than trypsinogen. The fourth variant, DeltaI16V17 trypsinogen, is the lone outlier in the correlations; its activity is lower than expected based on its affinity for BPTI. The S1 site and oxyanion hole, formed by segments 184A-194 and 216-223, are trypsinlike in all of the enzymes. These structural and kinetic data confirm that BPTI induces an active conformation in the trypsin(ogen) variants. Thus, changes in BPTI affinity monitor changes in the energetic cost of inducing a trypsinlike conformation. Although the S1 site and oxyanion hole are similar in all four variants, the N-terminal and autolysis loop (residues 142-152) segments have different interactions for each variant. These results indicate that zymogen activity is controlled by a simple conformational equilibrium between active and inactive conformations, and that the autolysis loop and N-terminal segments control this equilibrium. Together, these data illustrate that induced fit does not generally contribute to enzyme specificity.
Organizational Affiliation:
Department of Chemistry, Brandeis University, Waltham, Massachusetts 02454, USA.