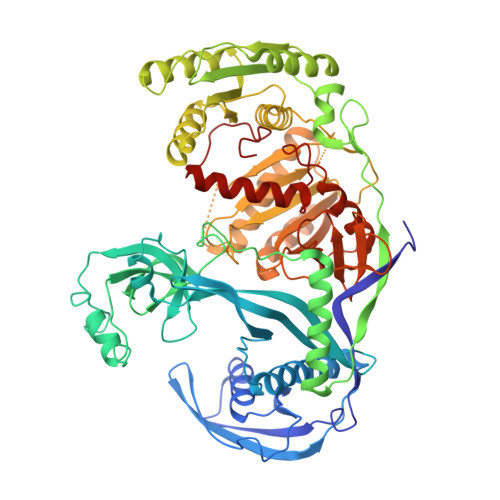
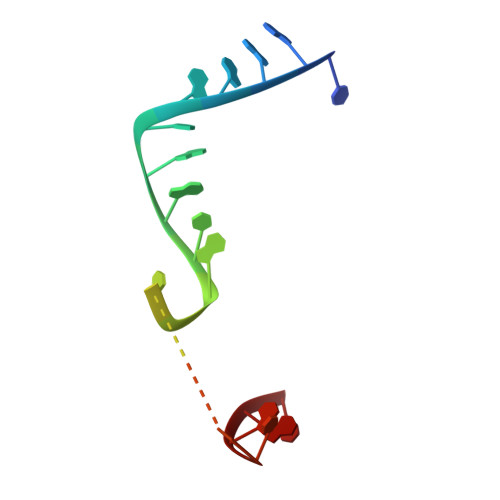
Structure of the guide-strand-containing argonaute silencing complex.
Wang, Y., Sheng, G., Juranek, S., Tuschl, T., Patel, D.J.(2008) Nature 456: 209-213
- PubMed: 18754009
- DOI: https://doi.org/10.1038/nature07315
- Primary Citation of Related Structures:
3DLB, 3DLH - PubMed Abstract:
The slicer activity of the RNA-induced silencing complex is associated with argonaute, the RNase H-like PIWI domain of which catalyses guide-strand-mediated sequence-specific cleavage of target messenger RNA. Here we report on the crystal structure of Thermus thermophilus argonaute bound to a 5'-phosphorylated 21-base DNA guide strand, thereby identifying the nucleic-acid-binding channel positioned between the PAZ- and PIWI-containing lobes, as well as the pivot-like conformational changes associated with complex formation. The bound guide strand is anchored at both of its ends, with the solvent-exposed Watson-Crick edges of stacked bases 2 to 6 positioned for nucleation with the mRNA target, whereas two critically positioned arginines lock bases 10 and 11 at the cleavage site into an unanticipated orthogonal alignment. Biochemical studies indicate that key amino acid residues at the active site and those lining the 5'-phosphate-binding pocket made up of the Mid domain are critical for cleavage activity, whereas alterations of residues lining the 2-nucleotide 3'-end-binding pocket made up of the PAZ domain show little effect.
Organizational Affiliation:
Structural Biology Program, Memorial Sloan-Kettering Cancer Center, New York, New York 10065, USA.