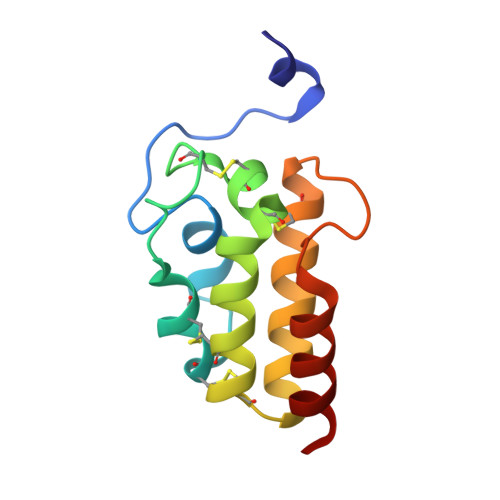
Structural basis of haem-iron acquisition by fungal pathogens.
Nasser, L., Weissman, Z., Pinsky, M., Amartely, H., Dvir, H., Kornitzer, D.(2016) Nat Microbiol 1: 16156-16156
- PubMed: 27617569
- DOI: https://doi.org/10.1038/nmicrobiol.2016.156
- Primary Citation of Related Structures:
4Y7S - PubMed Abstract:
Pathogenic microorganisms must cope with extremely low free-iron concentrations in the host's tissues. Some fungal pathogens rely on secreted haemophores that belong to the Common in Fungal Extracellular Membrane (CFEM) protein family, to extract haem from haemoglobin and to transfer it to the cell's interior, where it can serve as a source of iron. Here we report the first three-dimensional structure of a CFEM protein, the haemophore Csa2 secreted by Candida albicans. The CFEM domain adopts a novel helical-basket fold that consists of six α-helices, and is uniquely stabilized by four disulfide bonds formed by its eight signature cysteines. The planar haem molecule is bound between a flat hydrophobic platform located on top of the helical basket and a peripheral N-terminal 'handle' extension. Exceptionally, an aspartic residue serves as the CFEM axial ligand, and so confers coordination of Fe 3+ haem, but not of Fe 2+ haem. Histidine substitution mutants of this conserved Asp acquired Fe 2+ haem binding and retained the capacity to extract haem from haemoglobin. However, His-substituted CFEM proteins were not functional in vivo and showed disturbed haem exchange in vitro, which suggests a role for the oxidation-state-specific Asp coordination in haem acquisition by CFEM proteins.
- B. Rappaport Faculty of Medicine, Technion - Israel Institute of Technology, and the Rappaport Institute for Research in the Medical Sciences, Haifa 31096, Israel.
Organizational Affiliation: