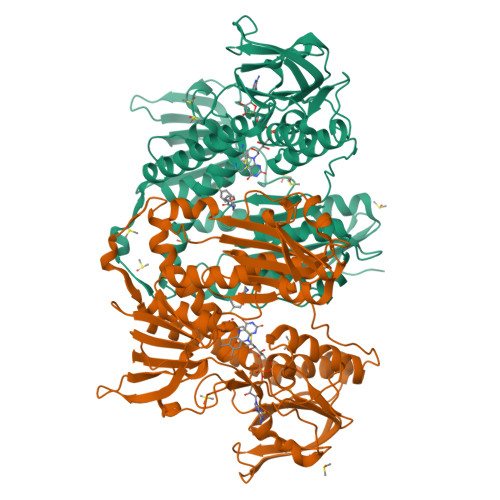
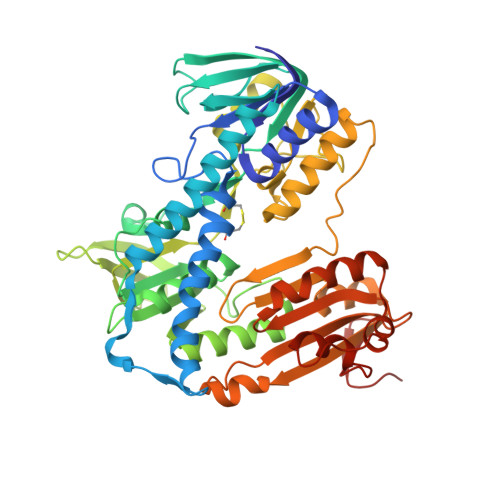
Innovative Approach for a Classic Target: Fragment Screening on Trypanothione Reductase Reveals New Opportunities for Drug Design.
Fiorillo, A., Colotti, G., Exertier, C., Liuzzi, A., Seghetti, F., Salerno, A., Caciolla, J., Ilari, A.(2022) Front Mol Biosci 9: 900882-900882
- PubMed: 35860359
- DOI: https://doi.org/10.3389/fmolb.2022.900882
- Primary Citation of Related Structures:
5S9S, 5S9T, 5S9U, 5S9V, 5S9W, 5S9X, 5S9Y, 5S9Z, 5SA0, 5SA1, 5SA2, 5SA3, 5SMJ - PubMed Abstract:
Trypanothione reductase (TR) is a key factor in the redox homeostasis of trypanosomatid parasites, critical for survival in the hostile oxidative environment generated by the host to fight infection. TR is considered an attractive target for the development of new trypanocidal agents as it is essential for parasite survival but has no close homolog in humans. However, the high efficiency and turnover of TR challenging targets since only potent inhibitors, with nanomolar IC50, can significantly affect parasite redox state and viability. To aid the design of effective compounds targeting TR, we performed a fragment-based crystal screening at the Diamond Light Source XChem facility using a library optimized for follow-up synthesis steps. The experiment, allowing for testing over 300 compounds, resulted in the identification of 12 new ligands binding five different sites. Interestingly, the screening revealed the existence of an allosteric pocket close to the NADPH binding site, named the "doorstop pocket" since ligands binding at this site interfere with TR activity by hampering the "opening movement" needed to allow cofactor binding. The second remarkable site, known as the Z-site, identified by the screening, is located within the large trypanothione cavity but corresponds to a region not yet exploited for inhibition. The fragments binding to this site are close to each other and have some remarkable features making them ideal for follow-up optimization as a piperazine moiety in three out of five fragments.
Organizational Affiliation:
Institute of Molecular Biology and Pathology, Italian National Research Council, IBPM-CNR, Rome, Italy.