Cytochrome c oxidase structure in O-state
Kolbe, F., Safarian, S., Michel, H.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome c oxidase subunit 1-beta | 558 | Paracoccus denitrificans | Mutation(s): 0 EC: 7.1.1.9 Membrane Entity: Yes | 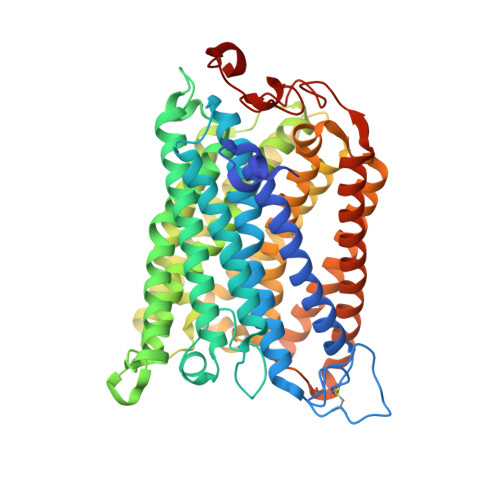 | |
UniProt | |||||
Find proteins for P98002 (Paracoccus denitrificans) Explore P98002 Go to UniProtKB: P98002 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P98002 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome c oxidase subunit 2 | 298 | Paracoccus denitrificans | Mutation(s): 0 EC: 7.1.1.9 Membrane Entity: Yes | 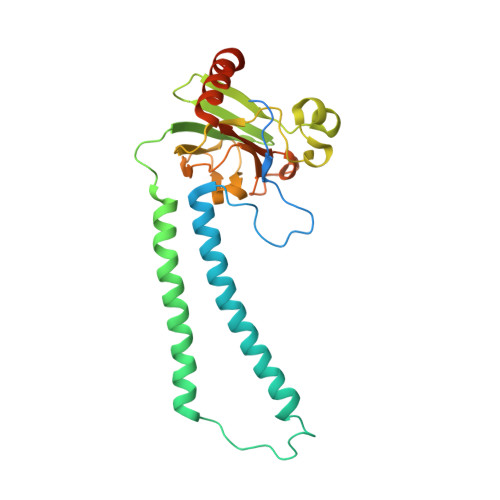 | |
UniProt | |||||
Find proteins for P08306 (Paracoccus denitrificans) Explore P08306 Go to UniProtKB: P08306 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P08306 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome c oxidase subunit 3 | 274 | Paracoccus denitrificans | Mutation(s): 0 EC: 7.1.1.9 Membrane Entity: Yes | 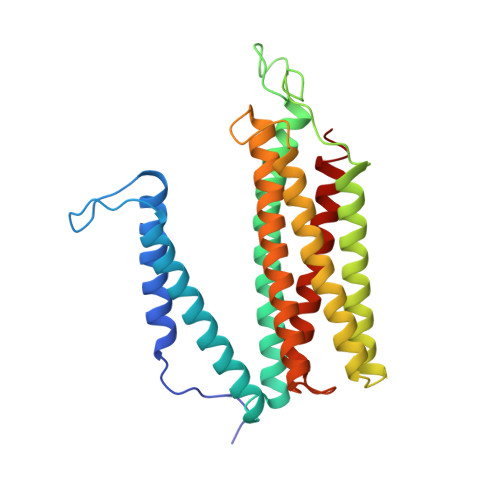 | |
UniProt | |||||
Find proteins for P06030 (Paracoccus denitrificans) Explore P06030 Go to UniProtKB: P06030 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P06030 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 4 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cytochrome c oxidase subunit 4 | 50 | Paracoccus denitrificans | Mutation(s): 0 EC: 7.1.1.9 Membrane Entity: Yes | 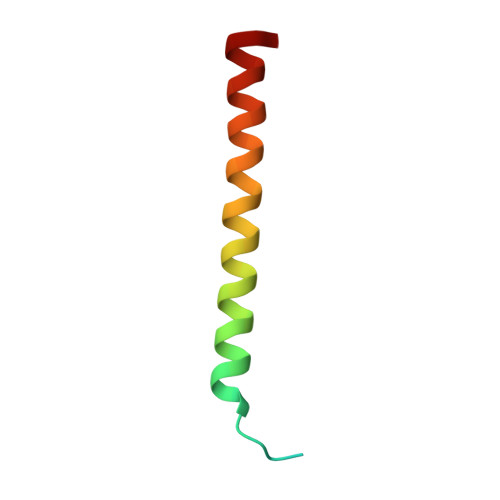 | |
UniProt | |||||
Find proteins for P77921 (Paracoccus denitrificans) Explore P77921 Go to UniProtKB: P77921 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P77921 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 8 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEA (Subject of Investigation/LOI) Query on HEA | E [auth A], F [auth A] | HEME-A C49 H56 Fe N4 O6 ZGGYGTCPXNDTRV-PRYGPKJJSA-L |  | ||
| PC1 Query on PC1 | R [auth C], S [auth C] | 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE C44 H88 N O8 P NRJAVPSFFCBXDT-HUESYALOSA-N |  | ||
| CUA Query on CUA | Q [auth B] | DINUCLEAR COPPER ION Cu2 ALKZAGKDWUSJED-UHFFFAOYSA-N |  | ||
| CU Query on CU | G [auth A] | COPPER (II) ION Cu JPVYNHNXODAKFH-UHFFFAOYSA-N |  | ||
| MN Query on MN | P [auth A] | MANGANESE (II) ION Mn WAEMQWOKJMHJLA-UHFFFAOYSA-N |  | ||
| CA Query on CA | H [auth A] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| PEO Query on PEO | O [auth A] | HYDROGEN PEROXIDE H2 O2 MHAJPDPJQMAIIY-UHFFFAOYSA-N |  | ||
| OXY Query on OXY | I [auth A] J [auth A] K [auth A] L [auth A] M [auth A] | OXYGEN MOLECULE O2 MYMOFIZGZYHOMD-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | Coot | 0.9 |
| RECONSTRUCTION | RELION | 3.1 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Max Planck Society | Germany | -- |