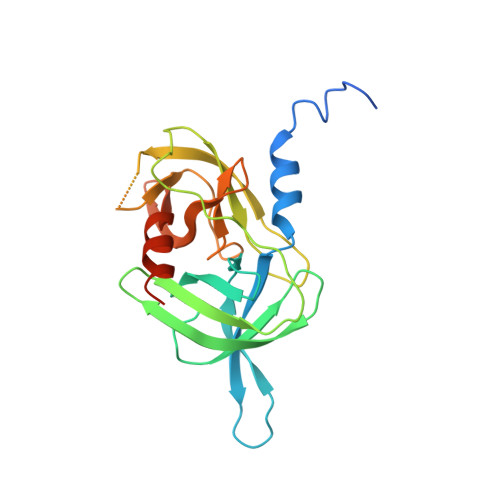
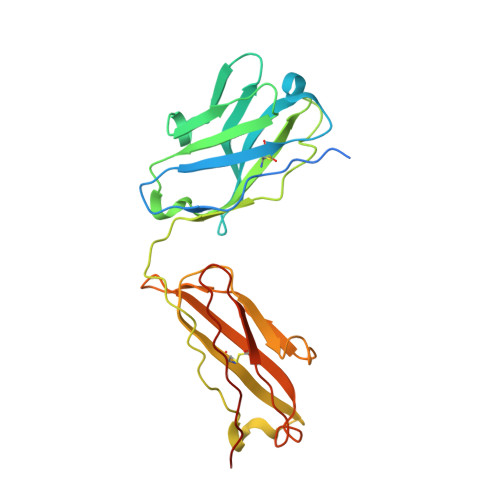
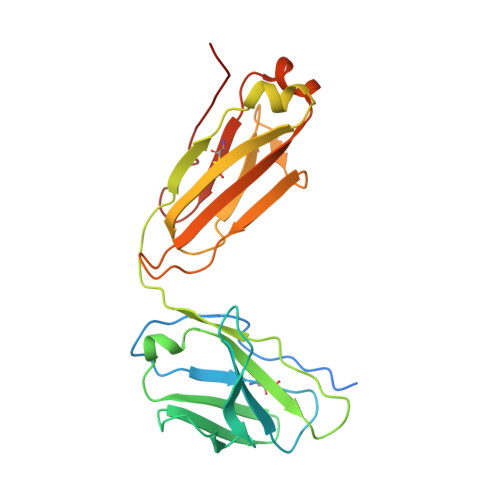
Allosteric inhibition of HTRA1 activity by a conformational lock mechanism to treat age-related macular degeneration.
Gerhardy, S., Ultsch, M., Tang, W., Green, E., Holden, J.K., Li, W., Estevez, A., Arthur, C., Tom, I., Rohou, A., Kirchhofer, D.(2022) Nat Commun 13: 5222-5222
- PubMed: 36064790
- DOI: https://doi.org/10.1038/s41467-022-32760-9
- Primary Citation of Related Structures:
7SJM, 7SJN, 7SJO, 7SJP - PubMed Abstract:
The trimeric serine protease HTRA1 is a genetic risk factor associated with geographic atrophy (GA), a currently untreatable form of age-related macular degeneration. Here, we describe the allosteric inhibition mechanism of HTRA1 by a clinical Fab fragment, currently being evaluated for GA treatment. Using cryo-EM, X-ray crystallography and biochemical assays we identify the exposed LoopA of HTRA1 as the sole Fab epitope, which is approximately 30 Å away from the active site. The cryo-EM structure of the HTRA1:Fab complex in combination with molecular dynamics simulations revealed that Fab binding to LoopA locks HTRA1 in a non-competent conformational state, incapable of supporting catalysis. Moreover, grafting the HTRA1-LoopA epitope onto HTRA2 and HTRA3 transferred the allosteric inhibition mechanism. This suggests a conserved conformational lock mechanism across the HTRA family and a critical role of LoopA for catalysis, which was supported by the reduced activity of HTRA1-3 upon LoopA deletion or perturbation. This study reveals the long-range inhibition mechanism of the clinical Fab and identifies an essential function of the exposed LoopA for activity of HTRA family proteases.
- Department of Early Discovery Biochemistry, Genentech Inc., San Francisco, CA, USA.
Organizational Affiliation: