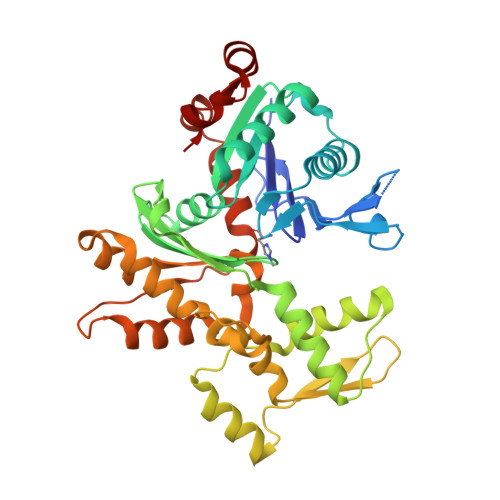
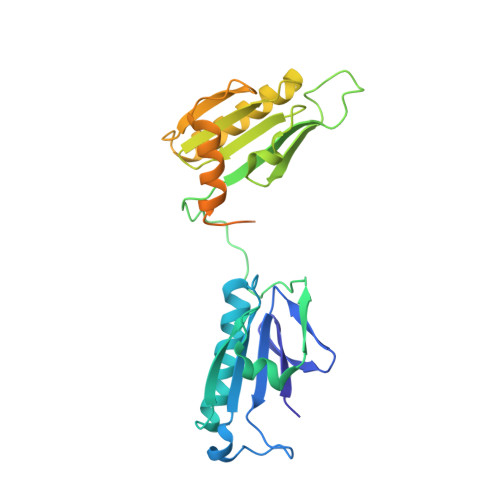
Structural and biochemical evidence for the emergence of a calcium-regulated actin cytoskeleton prior to eukaryogenesis
Akil, C., Tran, L.T., Orhant-Prioux, M., Baskaran, Y., Senju, Y., Takeda, S., Chotchuang, P., Muengsaen, D., Schulte, A., Manser, E., Blanchoin, L., Robinson, R.C.(2022) Commun Biol 5: 890