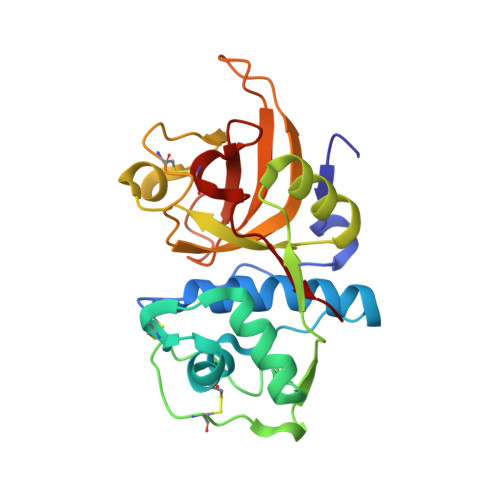
Structural Elucidation and Antiviral Activity of Covalent Cathepsin L Inhibitors.
Falke, S., Lieske, J., Herrmann, A., Loboda, J., Karnicar, K., Gunther, S., Reinke, P.Y.A., Ewert, W., Usenik, A., Lindic, N., Sekirnik, A., Dretnik, K., Tsuge, H., Turk, V., Chapman, H.N., Hinrichs, W., Ebert, G., Turk, D., Meents, A.(2024) J Med Chem 67: 7048-7067
- PubMed: 38630165
- DOI: https://doi.org/10.1021/acs.jmedchem.3c02351
- Primary Citation of Related Structures:
7QKD, 7ZS7, 7ZVF, 7ZXA, 8A4U, 8A4V, 8A4W, 8A4X, 8A5B, 8AHV, 8B4F, 8C77, 8OFA, 8OZA, 8PRX, 8QKB - PubMed Abstract:
Emerging RNA viruses, including SARS-CoV-2, continue to be a major threat. Cell entry of SARS-CoV-2 particles via the endosomal pathway involves cysteine cathepsins. Due to ubiquitous expression, cathepsin L (CatL) is considered a promising drug target in the context of different viral and lysosome-related diseases. We characterized the anti-SARS-CoV-2 activity of a set of carbonyl- and succinyl epoxide-based inhibitors, which were previously identified as inhibitors of cathepsins or related cysteine proteases. Calpain inhibitor XII, MG-101, and CatL inhibitor IV possess antiviral activity in the very low nanomolar EC 50 range in Vero E6 cells and inhibit CatL in the picomolar K i range. We show a relevant off-target effect of CatL inhibition by the coronavirus main protease α-ketoamide inhibitor 13b. Crystal structures of CatL in complex with 14 compounds at resolutions better than 2 Å present a solid basis for structure-guided understanding and optimization of CatL inhibitors toward protease drug development.
- Center for Free-Electron Laser Science CFEL, Deutsches Elektronen-Synchrotron DESY, Notkestraße 85, 22607 Hamburg, Germany.
Organizational Affiliation: