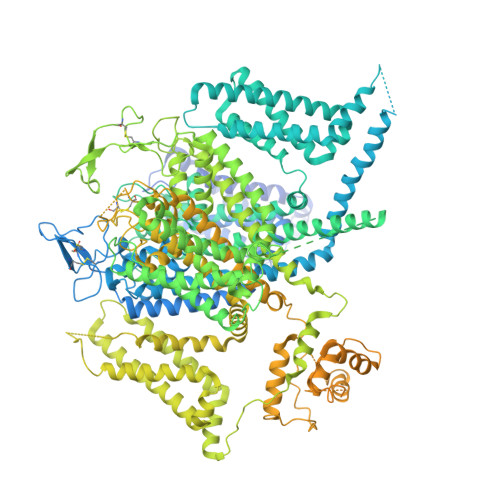
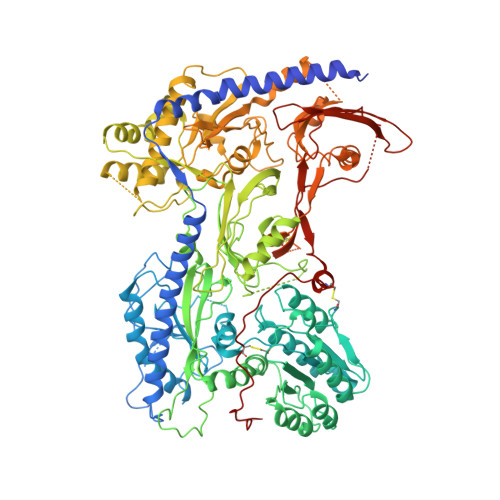
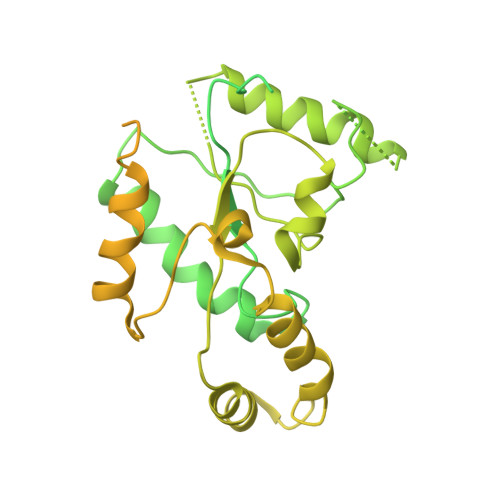
Structural bases of inhibitory mechanism of Ca V 1.2 channel inhibitors.
Wei, Y., Yu, Z., Wang, L., Li, X., Li, N., Bai, Q., Wang, Y., Li, R., Meng, Y., Xu, H., Wang, X., Dong, Y., Huang, Z., Zhang, X.C., Zhao, Y.(2024) Nat Commun 15: 2772-2772
- PubMed: 38555290
- DOI: https://doi.org/10.1038/s41467-024-47116-8
- Primary Citation of Related Structures:
8HLP, 8HMA, 8HMB - PubMed Abstract:
The voltage-gated calcium channel Ca V 1.2 is essential for cardiac and vessel smooth muscle contractility and brain function. Accumulating evidence demonstrates that malfunctions of Ca V 1.2 are involved in brain and heart diseases. Pharmacological inhibition of Ca V 1.2 is therefore of therapeutic value. Here, we report cryo-EM structures of Ca V 1.2 in the absence or presence of the antirheumatic drug tetrandrine or antihypertensive drug benidipine. Tetrandrine acts as a pore blocker in a pocket composed of S6 II , S6 III , and S6 IV helices and forms extensive hydrophobic interactions with Ca V 1.2. Our structure elucidates that benidipine is located in the D III -D IV fenestration site. Its hydrophobic sidechain, phenylpiperidine, is positioned at the exterior of the pore domain and cradled within a hydrophobic pocket formed by S5 DIII , S6 DIII , and S6 DIV helices, providing additional interactions to exert inhibitory effects on both L-type and T-type voltage gated calcium channels. These findings provide the structural foundation for the rational design and optimization of therapeutic inhibitors of voltage-gated calcium channels.
- Key Laboratory of Biomacromolecules (CAS), National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China.
Organizational Affiliation: