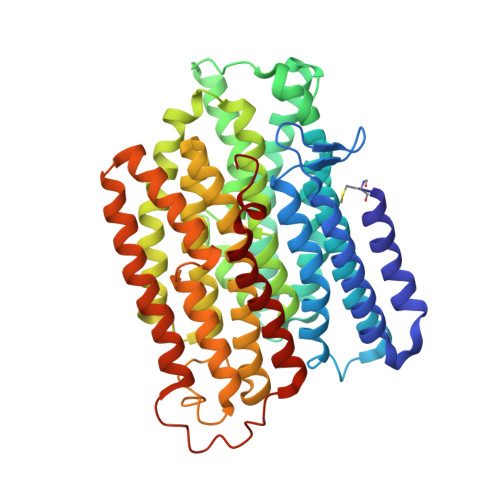
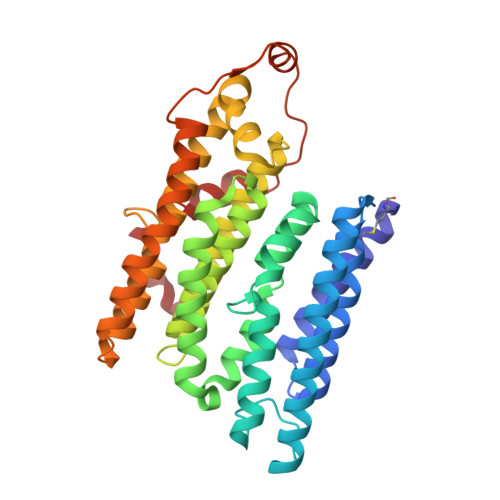
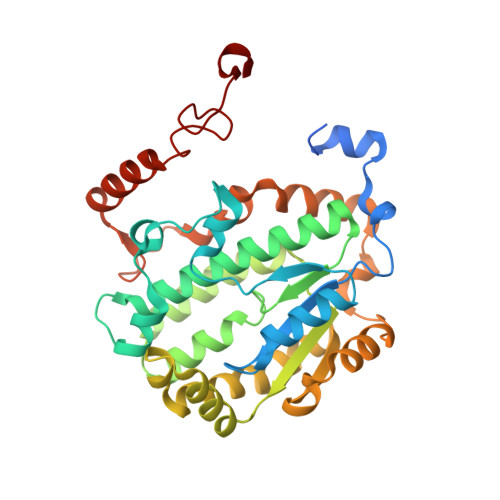
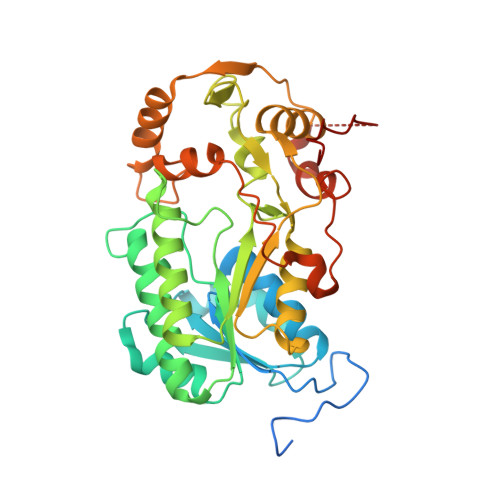
Molecular mechanism of the ischemia-induced regulatory switch in mammalian complex I.
Grba, D.N., Wright, J.J., Yin, Z., Fisher, W., Hirst, J.(2024) Science 384: 1247-1253
- PubMed: 38870289
- DOI: https://doi.org/10.1126/science.ado2075
- Primary Citation of Related Structures:
8Q0A, 8Q0F, 8Q0J, 8Q0M, 8Q0O, 8Q0Q, 8Q1P, 8Q1U, 8Q1Y, 8Q25, 8Q45, 8Q46, 8Q47, 8Q48, 8Q49, 8Q4A - PubMed Abstract:
Respiratory complex I is an efficient driver for oxidative phosphorylation in mammalian mitochondria, but its uncontrolled catalysis under challenging conditions leads to oxidative stress and cellular damage. Ischemic conditions switch complex I from rapid, reversible catalysis into a dormant state that protects upon reoxygenation, but the molecular basis for the switch is unknown. We combined precise biochemical definition of complex I catalysis with high-resolution cryo-electron microscopy structures in the phospholipid bilayer of coupled vesicles to reveal the mechanism of the transition into the dormant state, modulated by membrane interactions. By implementing a versatile membrane system to unite structure and function, attributing catalytic and regulatory properties to specific structural states, we define how a conformational switch in complex I controls its physiological roles.
- Medical Research Council Mitochondrial Biology Unit, University of Cambridge, Keith Peters Building, Cambridge Biomedical Campus, Hills Road, Cambridge CB2 0XY, UK.
Organizational Affiliation: