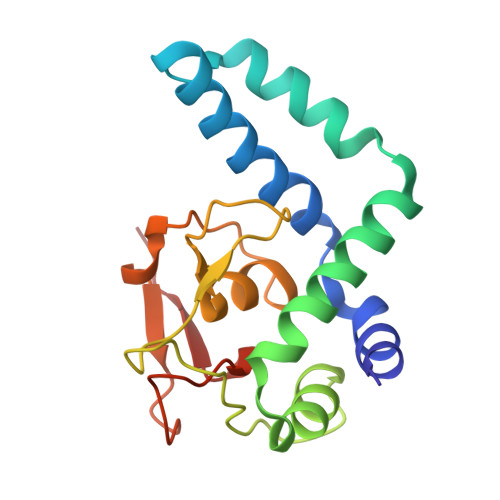
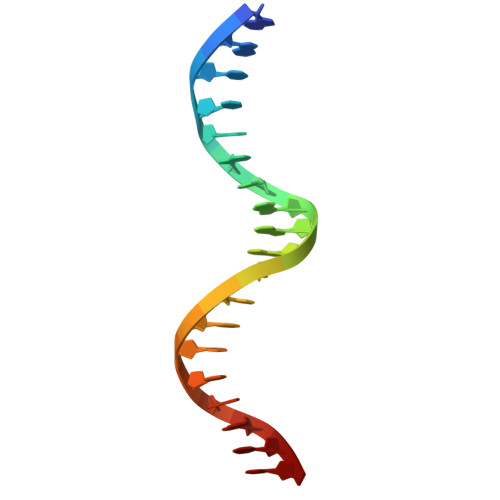
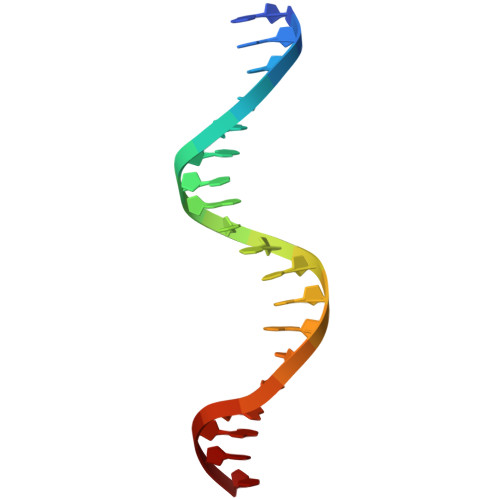
Molecular basis of NFIB-mediated regulation of oncogenic transcription.
Zhu, C., Xiao, D., Wang, Y., Han, H., Qin, C., Liu, S., Chen, X., Xiao, H., Chen, X., Shi, J., Tang, J., Shen, J., Song, H.(2025) Nucleic Acids Res 53
- PubMed: 41428734
- DOI: https://doi.org/10.1093/nar/gkaf1369
- Primary Citation of Related Structures:
9W7S, 9W7W - PubMed Abstract:
The Nuclear Factor I (NFI) family of transcription factors orchestrates key regulatory programs in development, differentiation, and metabolism, with dysregulation implicated in diverse pathological conditions, including cancer. Among the paralogs, NFIB has emerged as an oncogenic driver in multiple tumor types, yet the mechanisms through which it engages DNA and directs oncogenic transcriptional programs remain undefined. Here, using cancer cells with high NFIB expression, we demonstrate that NFIB promotes malignant phenotypes, as CRISPR-Cas9 knockout impairs proliferation, migration, and invasion. Transcriptomic profiling reveals that NFIB regulates a cancer-enriched gene network that includes FGFR3 and PDGFRB. Biophysical analyses show that NFIB, including its DNA-binding domain, functions as a monomer and binds DNA with strict 1:1 stoichiometry. High-resolution crystal structures of NFIB DNA-binding domain bound to ChIP-seq-derived DNA motifs reveal a monomeric binding mode mediated by conserved base-specific interactions with the TGGCA sequence, providing an atomic view of NFIB-DNA recognition. Mutational disruption of key DNA-contacting residues abolishes DNA binding and transcriptional activation, linking atomic-level recognition to oncogenic transcriptional regulation. Together, these findings elucidate the structural mechanism underlying NFIB function in cancer and establish a framework for therapeutic strategies targeting NFIB-driven malignancies.
- State Key Laboratory of Mechanism and Quality of Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau 999078, China.
Organizational Affiliation: