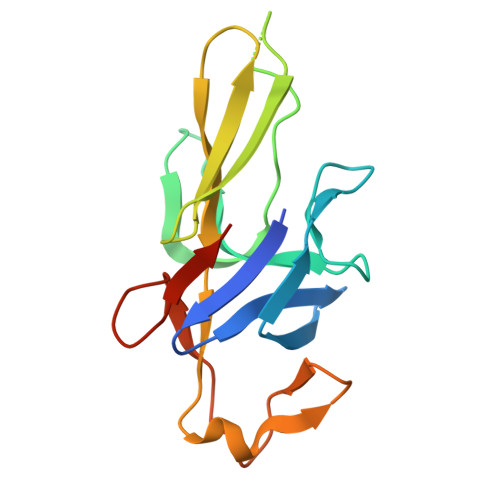
Structural characterization of Pseudomonas aeruginosa flagellar hook FlgE reveals a novel beta-hairpin element involved in inflammatory modulation.
You, Y., Lin, S., Yang, F., Chen, Z., Ye, F., Guo, Y., He, B., Cao, Y., Gu, J., Lu, G.(2025) Int J Biol Macromol 332: 148466-148466
- PubMed: 41130485
- DOI: https://doi.org/10.1016/j.ijbiomac.2025.148466
- Primary Citation of Related Structures:
9WWJ, 9WX3 - PubMed Abstract:
The flagellar hook subunit FlgE of Pseudomonas aeruginosa (Pa-FlgE) is a core component of the bacterial surface flagellum. It is also identified as an important virulence factor capable of modulating host inflammatory response. Herein, we report the high-resolution crystal structures of Pa-FlgE domain I (D-I) and domain II (D-II), both of which adopt predominantly β-structure. Structural comparison among Pa-FlgE and its orthologs shows that D-I and the core-barrel of D-II are highly conserved. Two peripheral insertions of variable length and structure, however, are identified in FlgE D-II. In Pa-FlgE, these two insertions fold as a loop element and a β-hairpin element, respectively. Notably, these two elements are solvent-exposed and extend towards one another, and deletion of the two elements either simultaneously or individually is shown to abolish the immunomodulation activity of Pa-FlgE. While the loop element is present in other FlgE orthologs, the β-hairpin is unique to Pa-FlgE, suggesting that P. aeruginosa has evolved this distinctive β-hairpin in flagellar hook to modulate the inflammatory response during infection.
- Department of Emergency Medicine, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, China.
Organizational Affiliation: