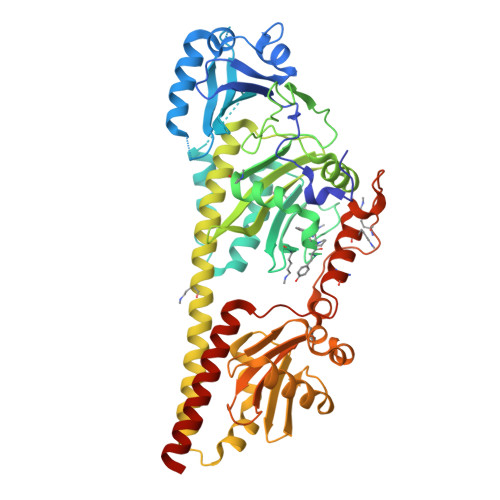
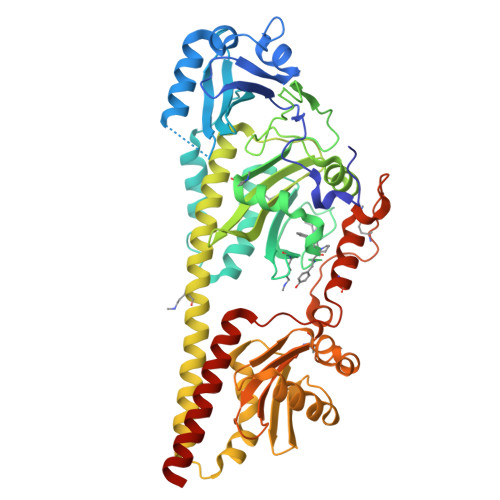
Hydrogen Bonding and Noncovalent Electric Field Effects in the Photoconversion of a Phytochrome.
Nguyen, A.D., Michael, N., Sauthof, L., von Sass, J., Hoang, O.T., Schmidt, A., La Greca, M., Schlesinger, R., Budisa, N., Scheerer, P., Mroginski, M.A., Kraskov, A., Hildebrandt, P.(2024) J Phys Chem B 128: 11644-11657
- PubMed: 39561028
- DOI: https://doi.org/10.1021/acs.jpcb.4c06419
- Primary Citation of Related Structures:
9G8C, 9G8D - PubMed Abstract:
A profound understanding of protein structure and mechanism requires dedicated experimental and theoretical tools to elucidate electrostatic and hydrogen bonding interactions in proteins. In this work, we employed an approach to disentangle noncovalent and hydrogen-bonding electric field changes during the reaction cascade of a multidomain protein, i.e., the phytochrome Agp2. The approach exploits the spectroscopic properties of nitrile probes commonly used as reporter groups of the vibrational Stark effect. These probes were introduced into the protein through site-specific incorporation of noncanonical amino acids resulting in four variants with different positions and orientations of the nitrile groups. All substitutions left structures and the reaction mechanism unchanged. Structural models of the dark states (Pfr) were used to evaluate the total electric field at the nitrile label and its transition dipole moment. These quantities served as an internal standard to calculate the respective properties of the photoinduced products (Lumi-F, Meta-F, and Pr) based on the relative intensities of the nitrile stretching bands. In most cases, the spectral analysis revealed two substates with a nitrile in a hydrogen-bonded or hydrophobic environment. Using frequencies and intensities, we managed to extract the noncovalent contribution of the electric field from the individual substates. This analysis resulted in profiles of the noncovalent and hydrogen-bond-related electric fields during the photoinduced reaction cascade of Agp2. These profiles, which vary significantly among the four variants due to the different positions and orientations of the nitrile probes, were discussed in the context of the molecular events along the Pfr → Pr reaction cascade.
Organizational Affiliation:
Institut für Chemie, Sekr. C7, Technische Universität Berlin, Straße des 17. Juni 115, Berlin D-10623, Germany.