Structural Insights into the Role of the C-Lobe of E6AP in Catalyzing K48-Linked Ubiquitin Chain Formation
Wu, X.W., Cai, H.Y., Du, Y.X., Liu, L.To be published.
Experimental Data Snapshot
Starting Models: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin-protein ligase E3A | 108 | Homo sapiens | Mutation(s): 0 Gene Names: UBE3A, E6AP, EPVE6AP, HPVE6A EC: 2.3.2.26 | 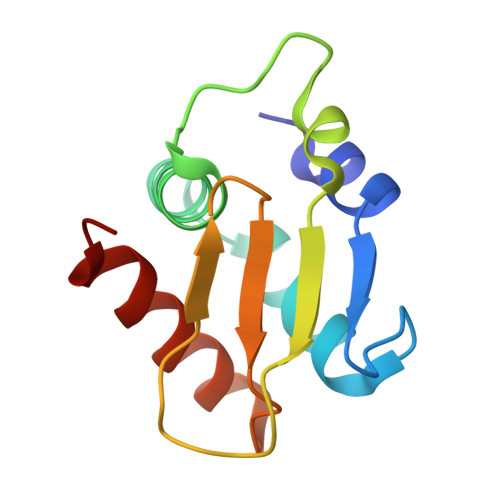 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q05086 (Homo sapiens) Explore Q05086 Go to UniProtKB: Q05086 | |||||
PHAROS: Q05086 GTEx: ENSG00000114062 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q05086 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Donor Ubiquitin | 76 | Homo sapiens | Mutation(s): 0 Gene Names: RPS27A, UBA80, UBCEP1 | 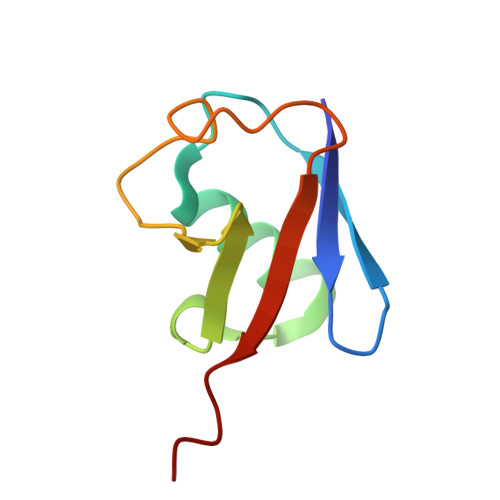 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P62979 (Homo sapiens) Explore P62979 Go to UniProtKB: P62979 | |||||
PHAROS: P62979 GTEx: ENSG00000143947 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62979 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Acceptor Ubiquitin | 73 | Homo sapiens | Mutation(s): 0 Gene Names: RPS27A, UBA80, UBCEP1 | 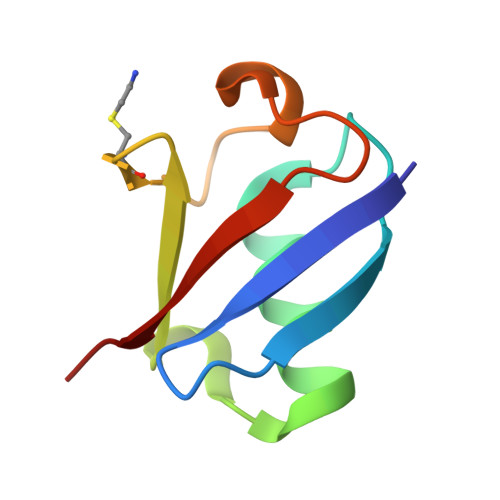 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P62979 (Homo sapiens) Explore P62979 Go to UniProtKB: P62979 | |||||
PHAROS: P62979 GTEx: ENSG00000143947 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P62979 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SLZ Query on SLZ | C | L-PEPTIDE LINKING | C5 H12 N2 O2 S |  | LYS |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 61.07 | α = 90 |
| b = 121.69 | β = 90 |
| c = 81.32 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 22137005 |
| National Natural Science Foundation of China (NSFC) | China | 92253302 |
| National Natural Science Foundation of China (NSFC) | China | 22227810 |