Isohelicity and phasing in drug--DNA sequence recognition: crystal structure of a tris(benzimidazole)--oligonucleotide complex.
Clark, G.R., Gray, E.J., Neidle, S., Li, Y.H., Leupin, W.(1996) Biochemistry 35: 13745-13752
- PubMed: 8901516
- DOI: https://doi.org/10.1021/bi960421m
- Primary Citation of Related Structures:
263D - PubMed Abstract:
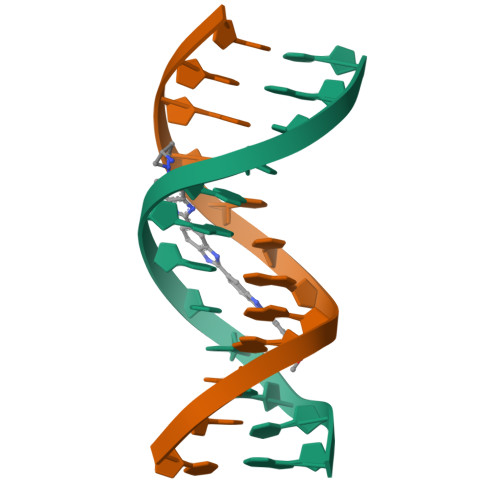
The crystal structure is reported of a tris(benzimidazole) analogue of the minor-groove drug Hoechst 33258 bound to the sequence d(CGCAAATTTGCG)2. The structure has been refined to an R factor of 17.4% at a resolution of 2.2 A. The ligand covers approximately 7 1/2 base pairs, including the 5'-AAATTT central sequence. This has an exceptionally narrow minor-groove width, together with high propeller twists for individual base pairs. The ligand has a highly twisted structure, with an overall twist of 50 degrees between aromatic rings. All three benzimidazole subunits are in register with the DNA, and there is a symmetric group of six hydrogen bonds between ligand and A.T base-pair edges. By contrast, the ligand does not show an optimal isohelical fit to the DNA. The correct phasing of drug and DNA base pairs is ensured by a number of changes to the DNA such that the central 5'-AAATTT region is slightly unwound relative to the structures of other noncovalent minor-groove drug complexes.
Organizational Affiliation:
CRC Biomolecular Structure Unit, Institute of Cancer Research, Sutton, Surrey, U.K.