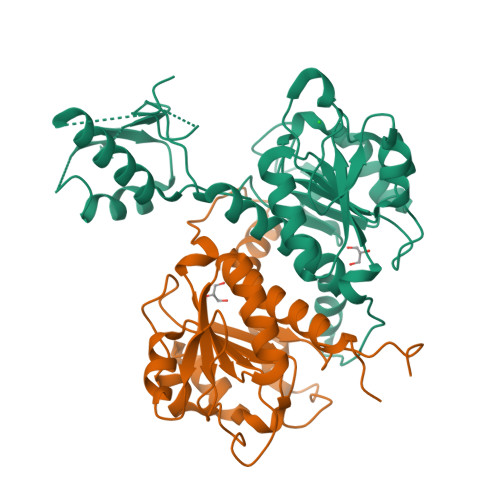
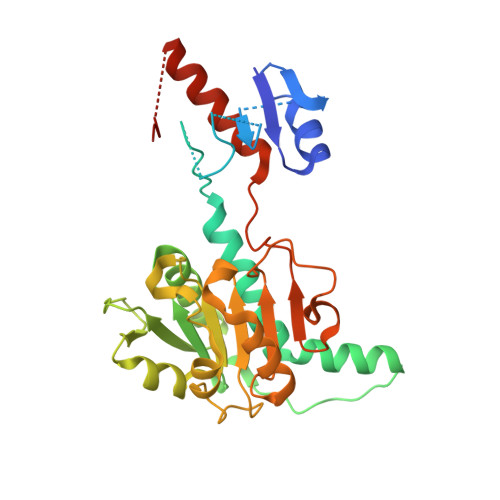
Structural, Biochemical, and Evolutionary Characterizations of Glyoxylate/Hydroxypyruvate Reductases Show Their Division into Two Distinct Subfamilies.
Kutner, J., Shabalin, I.G., Matelska, D., Handing, K.B., Gasiorowska, O., Sroka, P., Gorna, M.W., Ginalski, K., Wozniak, K., Minor, W.(2018) Biochemistry 57: 963-977
- PubMed: 29309127
- DOI: https://doi.org/10.1021/acs.biochem.7b01137
- Primary Citation of Related Structures:
4WEQ, 4Z0P, 5J23, 5UNN, 5UOG, 5V6Q, 5V72, 5V7G, 5V7N - PubMed Abstract:
The d-2-hydroxyacid dehydrogenase (2HADH) family illustrates a complex evolutionary history with multiple lateral gene transfers and gene duplications and losses. As a result, the exact functional annotation of individual members can be extrapolated to a very limited extent. Here, we revise the previous simplified view on the classification of the 2HADH family; specifically, we show that the previously delineated glyoxylate/hydroxypyruvate reductase (GHPR) subfamily consists of two evolutionary separated GHRA and GHRB subfamilies. We compare two representatives of these subfamilies from Sinorhizobium meliloti (SmGhrA and SmGhrB), employing a combination of biochemical, structural, and bioinformatics approaches. Our kinetic results show that both enzymes reduce several 2-ketocarboxylic acids with overlapping, but not equivalent, substrate preferences. SmGhrA and SmGhrB show highest activity with glyoxylate and hydroxypyruvate, respectively; in addition, only SmGhrB reduces 2-keto-d-gluconate, and only SmGhrA reduces pyruvate (with low efficiency). We present nine crystal structures of both enzymes in apo forms and in complexes with cofactors and substrates/substrate analogues. In particular, we determined a crystal structure of SmGhrB with 2-keto-d-gluconate, which is the biggest substrate cocrystallized with a 2HADH member. The structures reveal significant differences between SmGhrA and SmGhrB, both in the overall structure and within the substrate-binding pocket, offering insight into the molecular basis for the observed substrate preferences and subfamily differences. In addition, we provide an overview of all GHRA and GHRB structures complexed with a ligand in the active site.
Organizational Affiliation:
Department of Molecular Physiology and Biological Physics, University of Virginia , 1340 Jefferson Park Avenue, Charlottesville, Virginia 22908, United States.