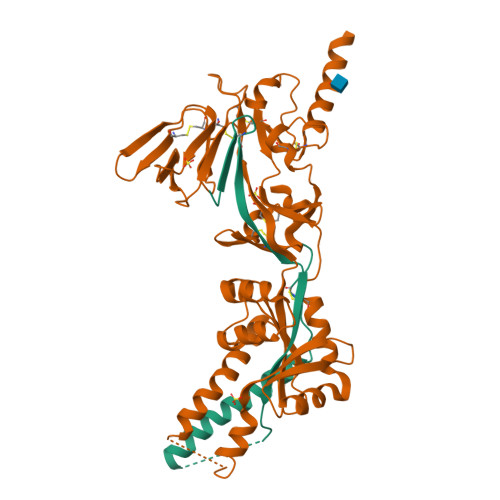
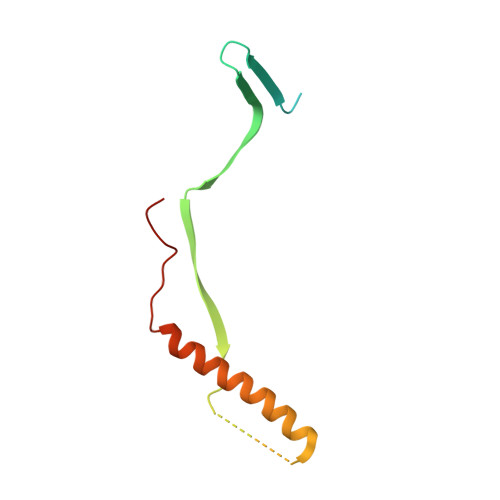
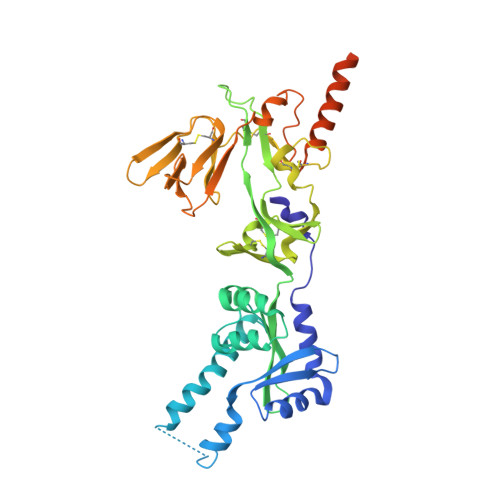
Rational design of a highly immunogenic prefusion-stabilized F glycoprotein antigen for a respiratory syncytial virus vaccine.
Che, Y., Gribenko, A.V., Song, X., Handke, L.D., Efferen, K.S., Tompkins, K., Kodali, S., Nunez, L., Prasad, A.K., Phelan, L.M., Ammirati, M., Yu, X., Lees, J.A., Chen, W., Martinez, L., Roopchand, V., Han, S., Qiu, X., DeVincenzo, J.P., Jansen, K.U., Dormitzer, P.R., Swanson, K.A.(2023) Sci Transl Med 15: eade6422-eade6422
- PubMed: 37023209
- DOI: https://doi.org/10.1126/scitranslmed.ade6422
- Primary Citation of Related Structures:
7UJ3, 7UJA - PubMed Abstract:
Respiratory syncytial virus (RSV) is the leading, global cause of serious respiratory disease in infants and is an important cause of respiratory illness in older adults. No RSV vaccine is currently available. The RSV fusion (F) glycoprotein is a key antigen for vaccine development, and its prefusion conformation is the target of the most potent neutralizing antibodies. Here, we describe a computational and experimental strategy for designing immunogens that enhance the conformational stability and immunogenicity of RSV prefusion F. We obtained an optimized vaccine antigen after screening nearly 400 engineered F constructs. Through in vitro and in vivo characterization studies, we identified F constructs that are more stable in the prefusion conformation and elicit ~10-fold higher serum-neutralizing titers in cotton rats than DS-Cav1. The stabilizing mutations of the lead construct (847) were introduced onto F glycoprotein backbones of strains representing the dominant circulating genotypes of the two major RSV subgroups, A and B. Immunization of cotton rats with a bivalent vaccine formulation of these antigens conferred complete protection against RSV challenge, with no evidence of disease enhancement. The resulting bivalent RSV prefusion F investigational vaccine has recently been shown to be efficacious against RSV disease in two pivotal phase 3 efficacy trials, one for passive protection of infants by immunization of pregnant women and the second for active protection of older adults by direct immunization.
Organizational Affiliation:
Discovery Sciences, Pfizer Inc, Groton, CT 06340, USA.