Design, quality and validation of the EU-OPENSCREEN fragment library poised to a high-throughput screening collection.
Jalencas, X., Berg, H., Espeland, L.O., Sreeramulu, S., Kinnen, F., Richter, C., Georgiou, C., Yadrykhinsky, V., Specker, E., Jaudzems, K., Miletic, T., Harmel, R., Gribbon, P., Schwalbe, H., Brenk, R., Jirgensons, A., Zaliani, A., Mestres, J.(2024) RSC Med Chem 15: 1176-1188
- PubMed: 38665834
- DOI: https://doi.org/10.1039/d3md00724c
- Primary Citation of Related Structures:
8PJ0, 8R0I, 8R1V - PubMed Abstract:
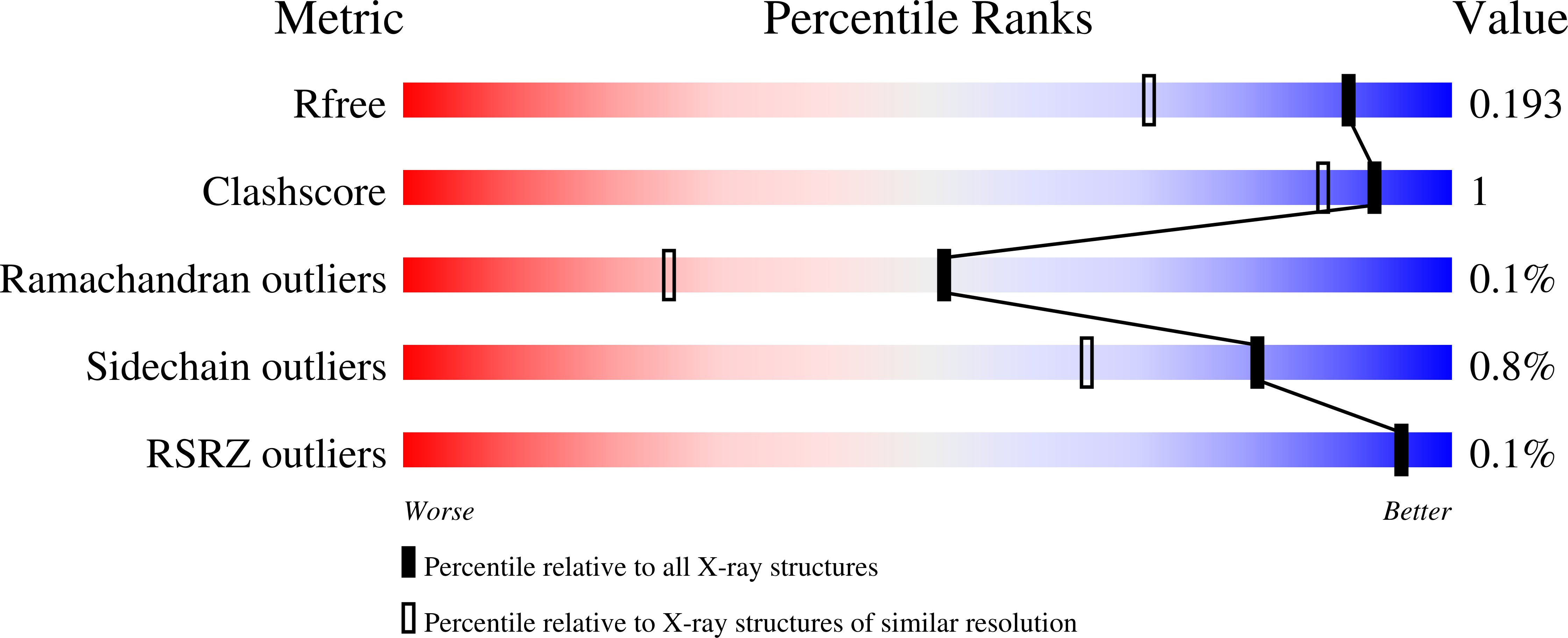
The EU-OPENSCREEN (EU-OS) European Research Infrastructure Consortium (ERIC) is a multinational, not-for-profit initiative that integrates high-capacity screening platforms and chemistry groups across Europe to facilitate research in chemical biology and early drug discovery. Over the years, the EU-OS has assembled a high-throughput screening compound collection, the European Chemical Biology Library (ECBL), that contains approximately 100 000 commercially available small molecules and a growing number of thousands of academic compounds crowdsourced through our network of European and non-European chemists. As an extension of the ECBL, here we describe the computational design, quality control and use case screenings of the European Fragment Screening Library (EFSL) composed of 1056 mini and small chemical fragments selected from a substructure analysis of the ECBL. Access to the EFSL is open to researchers from both academia and industry. Using EFSL, eight fragment screening campaigns using different structural and biophysical methods have successfully identified fragment hits in the last two years. As one of the highlighted projects for antibiotics, we describe the screening by Bio-Layer Interferometry (BLI) of the EFSL, the identification of a 35 μM fragment hit targeting the beta-ketoacyl-ACP synthase 2 (FabF), its binding confirmation to the protein by X-ray crystallography (PDB 8PJ0), its subsequent rapid exploration of its surrounding chemical space through hit-picking of ECBL compounds that contain the fragment hit as a core substructure, and the final binding confirmation of two follow-up hits by X-ray crystallography (PDB 8R0I and 8R1V).
Organizational Affiliation:
Research Group on Systems Pharmacology, Research Program on Biomedical Informatics (GRIB), IMIM Hospital del Mar Medical Research Institute Parc de Recerca Biomèdica (PRBB), Doctor Aiguader 88 08003 Barcelona Spain jmestres@imim.es.