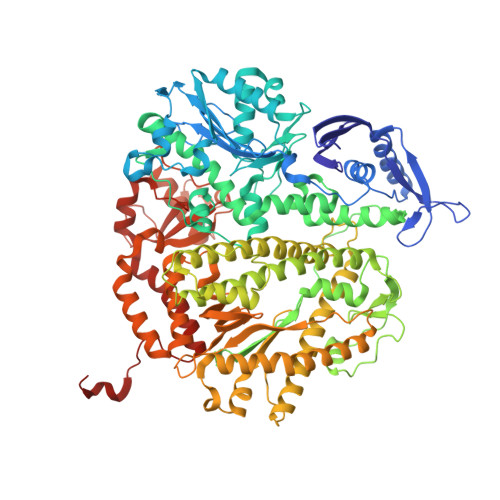
DNA mismatch synthesis complexes provide insights into base selectivity of a B family DNA polymerase.
Xia, S., Wang, J., Konigsberg, W.H.(2013) J Am Chem Soc 135: 193-202
- PubMed: 23214497
- DOI: https://doi.org/10.1021/ja3079048
- Primary Citation of Related Structures:
4FJ5, 4FJ7, 4FJ8, 4FJ9, 4FJG, 4FJH, 4FJI, 4FJJ, 4FJK, 4FJL, 4FJM, 4FJN, 4FJX, 4FK0, 4FK2, 4FK4 - PubMed Abstract:
Current hypotheses that attempt to rationalize the high degree of base selectivity exhibited by replicative DNA polymerases (pols) concur that ternary complexes formed with incorrect dNTPs are destabilized. Knowing what accounts for this destabilization is likely to be the key to understanding base discrimination. To address this issue, we have determined crystal structures of ternary complexes with all 12 mismatches using an engineered RB69 pol quadruple mutant (qm, L415A/L561A/S565G/Y567A) that enabled us to capture nascent mispaired dNTPs. These structures show that mismatches in the nascent base-pair binding pocket (NBP) of the qm pol differ markedly from mismatches embedded in binary pol-DNA complexes. Surprisingly, only 3 of 12 mismatches clash with the NBP when they are modeled into the wild-type (wt) pol. The remaining can fit into a wt pol ternary complex but deviate from normal Watson-Crick base-pairs. Repositioning of the templating nucleotide residue and the enlarged NBP in qm ternary complex play important roles in accommodating incorrect incoming dNTPs. From these structures, we propose additional reasons as to why incorrect dNTPs are incorporated so inefficiently by wt RB69 pol: (i) steric clashes with side chains in the NBP after Fingers closing; (ii) weak interactions or large gaps between the incoming dNTP and the templating base; and (iii) burying a protonated base in the hydrophobic environment of the NBP. All of these possibilities would be expected to destabilize the closed ternary complex so that incorporation of incorrect dNTP would be a rare event.
Organizational Affiliation:
Department of Molecular Biophysics and Biochemistry, Yale University, New Haven, Connecticut 06520, United States.