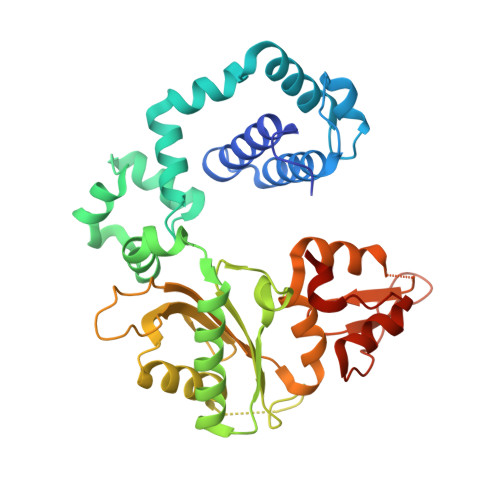
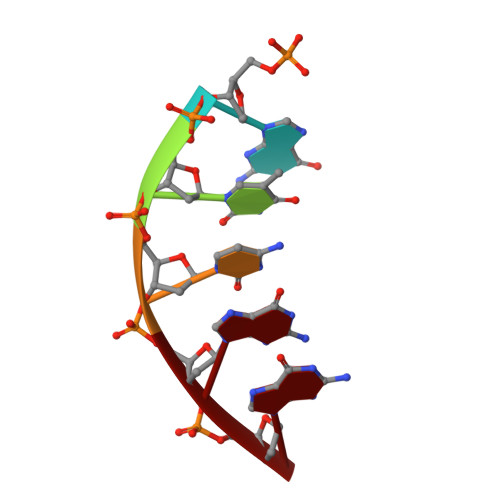
Structural basis for the D-stereoselectivity of human DNA polymerase beta.
Vyas, R., Reed, A.J., Raper, A.T., Zahurancik, W.J., Wallenmeyer, P.C., Suo, Z.(2017) Nucleic Acids Res 45: 6228-6237
- PubMed: 28402499
- DOI: https://doi.org/10.1093/nar/gkx252
- Primary Citation of Related Structures:
5U2R, 5U2S, 5U2T - PubMed Abstract:
Nucleoside reverse transcriptase inhibitors (NRTIs) with L-stereochemistry have long been an effective treatment for viral infections because of the strong D-stereoselectivity exhibited by human DNA polymerases relative to viral reverse transcriptases. The D-stereoselectivity of DNA polymerases has only recently been explored structurally and all three DNA polymerases studied to date have demonstrated unique stereochemical selection mechanisms. Here, we have solved structures of human DNA polymerase β (hPolβ), in complex with single-nucleotide gapped DNA and L-nucleotides and performed pre-steady-state kinetic analysis to determine the D-stereoselectivity mechanism of hPolβ. Beyond a similar 180° rotation of the L-nucleotide ribose ring seen in other studies, the pre-catalytic ternary crystal structures of hPolβ, DNA and L-dCTP or the triphosphate forms of antiviral drugs lamivudine ((-)3TC-TP) and emtricitabine ((-)FTC-TP) provide little structural evidence to suggest that hPolβ follows the previously characterized mechanisms of D-stereoselectivity. Instead, hPolβ discriminates against L-stereochemistry through accumulation of several active site rearrangements that lead to a decreased nucleotide binding affinity and incorporation rate. The two NRTIs escape some of the active site selection through the base and sugar modifications but are selected against through the inability of hPolβ to complete thumb domain closure.
Organizational Affiliation:
Department of Chemistry and Biochemistry, The Ohio State University, Columbus, OH 43210, USA.